1 Overview
In 1988, the nematode Pristionchus pacificus was isolated from garden soil in Pasadena, California (Sommer et al., 1996). P. pacificus was originally studied as a comparative species to Caenorhabditis elegans (PIntroFIG 1). Both species are easily reared in the laboratory on bacterial lawns and have similar anatomies and life cycles (Hong and Sommer, 2006). P. pacificus uses a facultative hermaphroditic reproductive strategy similar to C. elegans. Many of the laboratory tools developed for C. elegans research are readily transferable to P. pacificus (Pires-da Silva, 2013). The amenability of P. pacificus to genetic manipulation and analysis has propelled this species forward as an important comparative model for understanding the molecular basis of evolution for diverse traits.
PIntroFIG 1: Comparison of adult P. pacificus and C. elegans. Pristionchus pacificus (A) and Caenorhabditis elegans (B) adult hermaphrodites are shown by Nomarski DIC imaging (top images) and graphic illustration (bottom images). Both species have similar sizes and shapes. Scale bar 50 m.
Hundreds of P. pacificus isolates and other related Pristionchus species from around the world have been isolated (McGaughran and Morgan, 2015). Due to its cosmopolitan distribution and the diversity of isolated populations, P. pacificus has emerged as a powerful model for studying evolutionary processes at the molecular level. Many P. pacificus isolates are found associated with beetles and may have a necromenic relationship wherein the nematode waits for the beetle to die and then feeds off of the rotting cadaver and associated microorganisms (Herrmann et al., 2006). However, others have proposed that the relationship between P. pacificus and beetles is phoretic with the nematodes using beetles as a means of transport to a new nutrient rich environment (Félix et al., 2018).
P. pacificus is androdioecious, comprising both self-fertile hermaphrodites (XX) and occasional males (XO) that fertilize hermaphrodites. Males are usually less than 1% of the population; however, this fraction varies among P. pacificus isolates and environmental conditions (Morgan et al., 2017). The androdioecious system is valuable for genetic studies as the presence of hermaphrodites allows for the production of genetically identical offspring, while the presence of males allows for crosses between distinct genotypes.
2 P. pacificus Life Cycle
P. pacificus is easily maintained on bacterial cultures in the laboratory and frozen stocks can be kept for years (Pires-da Silva, 2013). Development from fertilized egg to adult is completed in approximately four days, which is only slightly longer than C. elegans (Hong and Sommer, 2006). Following exposure to harsh environmental conditions, P. pacificus can form a stress-resistant dauer stage that can survive extended periods without feeding.
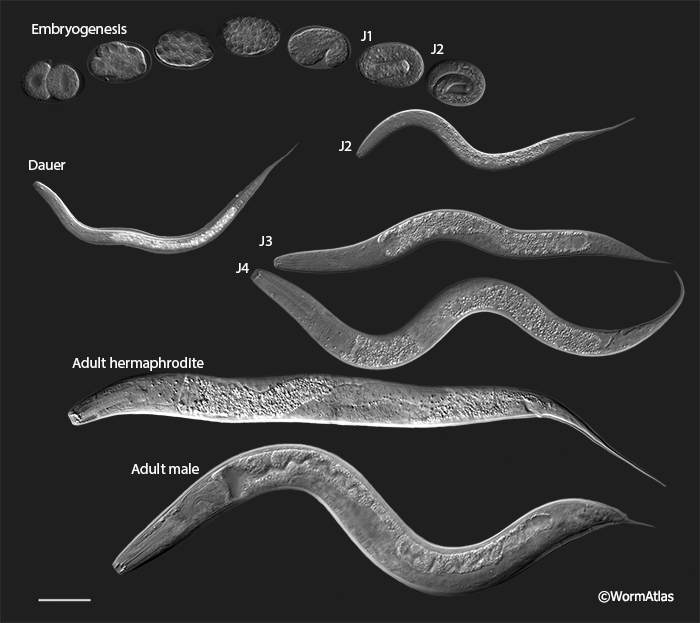
All nematodes, including C. elegans and P. pacificus, develop through four juvenile stages followed by the adult reproductive stage (PIntroFIG 2). Although identical in meaning, the juvenile stages of P. pacificus are known as J1-J4, while those of C. elegans are called L1-L4 (IntroFIG 8). Unlike C. elegans, which hatches as an L1/J1, P. pacificus does not emerge from its egg until L2/J2 (PIntroFIG 3). The presence of a distinct J1 stage in P. pacificus is marked by ecdysis (shedding of the cuticle) within the egg-shell (von Lieven, 2005) (PIntroFIG 3). While most species of nematodes hatch as J1s, the shift in timing of P. pacificus hatch may allow for the development of moveable teeth within the stoma. A similar delay is seen in many plant-parasitic nematodes with a moveable stylet mouth-part. Some events that occur in the post-hatch L1 of C. elegans occur prior to hatching in J1 P. pacificus. For example, migration of the Pn ectoblasts occur in the L1/J1 of both species (Sulston and Horvitz, 1977; Félix et al., 1999). Unfortunately, the additional post-embryonic development and corresponding movement within the eggshell will make it challenging to describe the complete embryonic cell lineage of P. pacificus.
PIntroFIG 3: P. pacificus J2 stage before hatching. The presence of a shed J1 cuticle (arrowhead) is indicative of the J1 to J2 molt occurring prior to hatching. Scale bar, 10 um.
3 Adult Anatomy
3.1 Body Shape
P. pacificus has a typical nematode body plan comprising a tube within a tube under hydrostatic pressure (PIntroFIG 1). The adult hermaphrodite P. pacificus is approximately 1 mm in length while the male is smaller and contains male sexual structures. Behaviors are driven by a nervous system that includes sensory, inter-, and motor neurons. In many cases, clear homology exists between individual cells in C. elegans and P. pacificus, including many neurons. Readers are encouraged to compare the tissue-specific descriptions in the C. elegans Handbook.
3.2 Organs and Tissues
3.2.1 Cuticle
The cuticle of P. pacificus is a tough rigid extracellular matrix secreted by the underlying epithelial cells. Similar to C. elegans and other nematodes (see C. elegans Cuticle), collagen is a major biochemical component of the body wall (Kenning et al., 2004; Schlager et al., 2009). The cuticle of P. pacificus contains evenly spaced stippled longitudinal ridges along the length of the body (PIntroFIG 4). Unlike the C. elegans alae, these longitudinal ridges are not restricted to the lateral field of the cuticle. Several tissues open to the outside through the cuticle including the stoma and anus openings to the digestive system. The excretory pore of P. pacificus lies on the ventral ridge near the terminal bulb of the pharynx. The lateral cuticle of P. pacificus hermaphrodites is also interrupted by a series of small pores that open to gland cells embedded within the lateral hypodermis (PIntroFIG 4).
A new layer of cuticle is produced at each molt, followed by the loosening and eventual shedding of the old cuticle. P. pacificus dauers tend to retain their L2 cuticle for extended periods of time. Retention of the L2 cuticle likely assists dauers in survival of adverse environmental conditions. P. pacificus dauers also secrete a waxy ester onto the cuticle surface that facilitates aggregation of dauers and the formation of ‘dauer towers’ (see Dauer Behavior for examples), which enhance the nematode’s ability to attach to a beetle host (Penkov et al., 2014).
PIntroFIG 4: The cuticle of P. pacificus. A. The cuticle of P. pacificus contains longitudinal stippled lines around the circumference of the animal. Scale bar, 10 um. B. In an EM cross section, this stippling can be seen as waves around the animal (arrowheads). Scale bar, 1 m. (Image source: Ralf Sommer Lab, Bumbarger13-1301.) C&D The cuticle of P. pacificus is interrupted by epithelial gland cell pores. In DIC (C), the small gland cell pores (arrow) are occasionally visible both dorsal and ventral of the midline (deirid, arrowhead). Scale bar, 10 µm. In EM cross sections (D), the epithelial gland cell (purple) is surrounded by the hypodermis. Scale bar, 1 m. (Image Source: Ralf Sommer, Bumbarger14-1955.)
3.2.2 Epithelial System
The main epithelial system of P. pacificus consists of hypodermal syncytia. Similar to C. elegans, the hypodermis wraps around the body wall of the nematode alternating between thick regions with nuclei in the cords and a thin sheet-like region underlying the somatic muscles (see C. elegans Hypodermis). The hypodermis is interrupted on the lateral ridges by a linear set of 15 epithelial ‘seam’ cells per side in the adult hermaphrodite, slightly less than the 16 homologous seam cells found in C. elegans (Cinkornpumin et al., 2014). Also, differing from C. elegans, the P. pacificus seam cells send processes from the apical membrane that extend away from the lateral midline (for more detail see C. elegans Seam).
3.2.3 Nervous System
Similar to C. elegans and other nematodes, the majority of P. pacificus neurons are located in the head and tail. The anterior nervous system of P. pacificus, including the pharynx and anterior amphid sensory neurons, have been reconstructed from serial-section electron microscopy (Bumbarger et al., 2013; Hong et al., 2019). The nervous system of P. pacificus is remarkably similar to that of C. elegans (see C. elegans Nervous System and Neuronal Support System). Both the anterior amphid sensory sensilla and the pharyngeal nervous system have equivalent numbers of neurons in both species and many of these are obvious homologs based on their positions and structures (Bumbarger et al., 2013; Bumbarger and Riebesell, 2015; Hong et al., 2019). While both species have 12 pairs of amphid neurons, the sensory ending shape differs between some homologous neurons. For example, the P. pacificus amphids lack obvious wing-like ciliated dendrites found in the AWA, AWB, and AWC neurons in C. elegans.
Similarly, the pharyngeal nervous system of both species comprises 20 neurons; however, synaptic connectivity differs between these species (Bumbarger et al., 2013) although a more recent reanalysis of the C. elegans pharyngeal connectome suggests these differences may not be as great as originally reported (Cook et al., 2020). Wiring differences between the two species in the amphid and pharyngeal circuits may mediate behavioral differences (Bumbarger et al., 2013; Hong et al., 2019). Similarly, slight differences in neurotransmitter expression occur between P. pacificus and C. elegans neurons (Loer and Rivard, 2007). While there are differences reported in the number of neurons within the ventral nerve cord between C. elegans and P. pacificus, additional electron microscopy will be needed to determine the extent of neuronal differences between these species (Han et al., 2016).
3.2.4 Muscle System
The somatic body wall muscle of P. pacificus is platymyarian, in which the obliquely striated contractile sarcomeres lie flat against the body wall, and is similar in overall structure to C. elegans (see C. elegans Somatic Muscle) (PIntroFIG 5). A basal lamina separates neurons and hypodermis from the body wall muscle. Typical to all nematodes, innervation of P. pacificus body wall muscles occurs through muscle arm processes extending to neurons. In addition to the body wall, non-striated muscles exist in the pharynx and surrounding the egg-laying apparatus and rectum.
PIntroFIG 5: P. pacificus body wall muscles. Transverse EM section through head showing muscles (green) lying against body wall. Scale bar, 1 m.
3.2.5 Excretory System
The P. pacificus excretory system appears very similar to the C. elegans excretory system (see C. elegans Excretory System). It consists of four cells: two excretory glands, a canal cell with bilaterally symmetrical longitudinal processes, and a duct cell (PIntroFIG 6). Similar to C. elegans, the P. pacificus CAN neuron travels alongside the canal cell processes (Carstensen et al., 2021). In P. pacificus, ablation of the CAN results in increased dauer formation, whereas in C. elegans ablation of CAN results in death (White et al., 1986; Mayer et al., 2015).
PIntroFIG 6: P. pacificus excretory system. Transverse TEM micrograph through junction of excretory glands with canal cell and secretory vesicle being released in gland cell ampulla (arrowhead). Scale bars, 1 m. (Image source: Ralf Sommer lab, Bumbarger13_2251.)
3.2.6 Coelomocyte System
Both C. elegans and P. pacificus adult hermaphrodites contain six large scavenger cells called coelomocytes that sit within the pseudocoelom body cavity (C. elegans Coelomocyte System) (Jungblut and Sommer, 1998). For both species, two of the coelomocytes are generated post-embryonically (Photos et al., 2006).
3.2.7 Alimentary System
The stoma of P. pacificus comprises a cuticular lined cavity. Consistent with many nematodes in the Diplogasteridae family, the P. pacificus stoma contains teeth used for predatory feeding of other nematodes (PIntroFIG 7). The morphology of the stoma varies between two morphotypes called eurystomatous and stenostomatous that are specialized for predatory vs. microbial feeding, respectively (Serobyan et al., 2014). The eurystomatous (wide-mouthed) is characterized by a claw-like dorsal tooth and an opposing subventral tooth. The stenostomatous (narrow-mouthed) form has a less prominent triangle-shaped dorsal tooth and lacks the additional subventral tooth. The specific morphotype is determined through environmental conditions and represents an example of a polyphenism (Bento et al., 2010; Serobyan et al., 2013). The teeth of P. pacificus are attached to the anterior most muscles of the pharynx, allowing for movement during feeding. Similar to C. elegans, the cuticle lined stoma is shed during each molt.
Posterior of the stoma, the P. pacificus pharynx is a muscular pump. While similar in gross morphology to the C. elegans pharynx (C. elegans Alimentary System - Pharynx), the P. pacificus pharynx uses a different pumping sequence to ingest food (Chiang et al., 2006). The P. pacificus pharynx lacks both a grinder and two gland cells found in the C. elegans terminal bulb; however, the three remaining gland cells of P. pacificus have evolved to occupy a larger fraction of the terminal bulb volume (Riebesell and Sommer, 2017).
The intestine connects to the posterior end of the pharynx. The lineage and a detailed description of the P. pacificus intestine are not yet available.
PIntroFIG 7: P. pacificus pharynx. A. Longitudinal EM through stoma of euryostomatous form with dorsal tooth (arrowheads). The dorsal tooth attaches to pharyngeal muscle (green) and contains an opening for vesicles from the dorsal pharyngeal gland (purple) to be released into the stoma. A subventral tooth (arrow) is seen on the opposite side. Scale bar, 1 m. B. Transverse EM micrograph through a euryostomatous stoma showing dorsal tooth (arrowhead) with lumen for dorsal pharyngeal gland secretions. A subventral tooth (arrow) is found in the subventral sector of the stoma. Scale bar, 1 m. (Image source: Ralf Sommer Lab, Bumbarger13-154.) C,D. DIC comparison of eurystomatous (C) and stenostomatous (D) forms. The eurystomatous form contains an obvious dorsal tooth (arrowhead). (Image source: Erik Ragsdale.)
3.2.8 Reproductive System
The first molecular genetic analyses of P. pacificus focused on vulva formation (Sommer and Sternberg, 1996). Similar to C. elegans, the reproductive system of P. pacificus consists of a somatic gonad, the germ line, and the egg-laying apparatus (for comparison see C. elegans Somatic Gonad, Germ Line and Egg-laying Apparatus). However, there is substantial divergence in the number of cells, overall shape and developmental timing of the reproductive systems between C. elegans and P. pacificus (Kolotuev and Podbilewicz, 2004; Rudel et al., 2005).
Similar to C. elegans, the P. pacificus vulva is formed through a combination of cell division, migration and fusion from three ectoblastic vulval precursor P cells; however, the molecular signal controlling division differs between the species (Sommer and Sternberg, 1996). Following induction, the P cells undergo divisions to form 20 vulval cells (two less than C. elegans) (Kolotuev and Podbilewicz, 2004). The vulval cells migrate to form a stack of rings at the location of the vulva and undergo cell-cell fusion to form stacked toroids. The timing, sequence and number of toroids differs between C. elegans and P. pacificus (Kolotuev and Podbilewicz, 2004).
The gonad of P. pacificus is didelphic, comprising two arms converging on a vulva positioned near the center of the body. The somatic gonad consists of a uterus, spermatheca, sheath, and distal tip cells. The germline is a syncytium connected through a central rachis. Several features of the gonad in P. pacificus distinguish it from that in C. elegans (Rudel et al., 2005). Most obviously distinct in the P. pacificus gonad is the pretzel shape of the gonad arms, which extend from the ventral side to the dorsal side and back again (PIntroFIG 8). The sheath cells wrap the proximal oocytes of both species, but differ in number between species (4 pairs in P. pacificus, 5 in C. elegans). While both species have sperm storage organs called spermatheca, P. pacificus does not contain the connective uterine-spermatheca valve cells found in C. elegans and differs in numbers of cells.
PIntroFIG 8: P. pacificus gonad. Gonad region of P. pacificus young adult shown via Nomarski DIC (top) and graphic illustration (bottom). Unlike in C. elegans, the distal arms of the P. pacificus gonad extend back to the ventral side. Scale bar, 10 m.
|
4 References
Bento, G., Ogawa, A., and Sommer, R.J. 2010. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 466: 494-7. doi: 10.1038/nature09164
Bumbarger, D.J. and Riebesell, M. 2015. Anatomy and connectivity in the pharyngeal nervous system. In: Pristionchus pacificus-A nematode model for comparative and evolutionary biology (Sommer RJ, Hunt DJ, Perry RN, eds), pp 353–383. Brill. doi: 10.1163/9789004260306_014
Bumbarger, D.J., Riebesell, M., Rödelsperger, C. and Sommer, R.J, 2013. System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes.. Cell 152: 109-19. doi: 10.1016/j.cell.2012.12.013
Carstensen, H.R., Villalon, R.M., Banerjee, N., Hallem, E.A. and Hong, R.L. 2021. Steroid hormone pathways coordinate developmental diapause and olfactory remodeling in Pristionchus pacificus. Genetics doi: 10.1093/genetics/iyab071
Chiang, J-T.A., Steciuk, M., Shtonda, B. and Avery, L. 2006. Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J. Exp. Biol. 209: 1859-1873. doi: 10.1242/jeb.02165
Cinkornpumin, J.K., Wisidagama, D.R., Rapoport, V., Go, J.L., Dieterich, C., Wang, X., Sommer, R.J. and Hong, R.L. 2014. A host beetle pheromone regulates development and behavior in the nematode Pristionchus pacificus. Elife 3: 1–21. doi: 10.7554/eLife.03229
Cook, S.J., Crouse, C.M., Yemini, E., Hall, D.H., Emmons, S.W. and Hobert, O. 2020. The connectome of the Caenorhabditis elegans pharynx. J. Comp. Neurol. 528: 2767-2784. doi: 10.1002/cne.24932
Félix, M-A., Hill, R.J., Schwarz, H., Sternberg, P.W., Sudhaus, W. and Sommer R.J. 1999. Pristionchus pacificus, a nematode with only three juvenile stages, displays major heterochronic changes relative to Caenorhabditis elegans. Proc. R. Soc. London Ser. B Biol. Sci. 266: 1617–21. doi: 10.1098/rspb.1999.0823
Félix, M-A., Ailion, M., Hsu, J-C., Richaud, A. and Wang, J. 2018. Pristionchus nematodes occur frequently in diverse rotting vegetal substrates and are not exclusively necromenic, while Panagrellus redivivoides is found specifically in rotting fruits. PLoS One 13:e0200851. doi: 10.1371/journal.pone.0200851
Han, Z., Boas, S. and Schroeder, N.E. 2016. Unexpected variation in neuroanatomy among diverse nematode species. Front. Neuroanat. 9: 162. doi: 10.3389/fnana.2015.00162
Herrmann, M., Mayer, W.E. and Sommer, R.J. 2006. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology 109: 96–108. doi: 10.1016/j.zool.2006.03.001
Hong, R.L., Riebesell, M., Bumbarger, D.J., Cook, S.J., Carstensen, H.R., Sarpolaki, T., Cochella, L., Castrejon, J., Moreno, E., Sieriebriennikov, B., Hobert, O. and Sommer, R.J. 2019. Evolution of neuronal anatomy and circuitry in two highly divergent nematode species. eLife 8: e47155. doi: 10.7554/eLife.47155
Hong, R.L. and Sommer, R.J. 2006. Pristionchus pacificus: a well-rounded nematode. Bioessays 28: 651–9. doi: 10.1002/bies.20404
Jungblut, B. and Sommer, R.J. 1998. The Pristionchus pacificus mab-5 gene is involved in the regulation of ventral epidermal cell fates. Curr. Biol. 8: 775–8. doi: 10.1016/s0960-9822(98)70301-x
Kenning, C., Kipping, I. and Sommer, R.J. 2004. Isolation of mutations with dumpy-like phenotypes and of collagen genes in the nematode Pristionchus pacificus. Genesis 40: 176–83. doi: 10.1002/gene.20084
Kolotuev, I. and Podbilewicz, B. 2004. Pristionchus pacificus vulva formation: Polarized division, cell migration, cell fusion, and evolution of invagination. Dev. Biol. 266: 322–33. doi: 10.1016/j.ydbio.2003.10.029
Loer, C.M. and Rivard, L. 2007. Evolution of neuronal patterning in free-living rhabditid nematodes I: Sex-specific serotonin-containing neurons. J. Comp. Neurol. 502: 736–67. doi: 10.1002/cne.21288
Mayer, M.G., Rödelsperger, C., Witte, H., Riebesell, M. and Sommer, R.J. 2015. The orphan gene dauerless regulates dauer development and intraspecific competition in nematodes by copy number variation. PLoS Genet. 11: e1005146. doi: 10.1371/journal.pgen.1005146
McGaughran, A. and Morgan, K. 2015. Population genetics and the La Reunion case study. In: Pristionchus pacificus - A nematode model for comparative and evolutionary biology (Sommer R.J. ed), pp 19719. doi: 10.1163/9789004260306_009
Morgan, K., McGaughran, A., Rdelsperger, C. and Sommer, R.J. 2017. Variation in rates of spontaneous male production and recombination within the nematode species P. pacificus supports an adaptive role for males and outcrossing. BMC Evol. Biol. 17: 57. doi: 10.1186/s12862-017-0873-7
Penkov, S., Ogawa, A., Schmidt, U., Tate, D., Zagoriy, V., Boland, S., Gruner, M., Vorkel, D., Verbavatz, J.M., Sommer, R.J., Knölker, H.J. and Kurzchalia, T.V. 2014. A wax ester promotes collective host finding in the nematode Pristionchus pacificus. Nat. Chem. Biol. 10: 281–5. doi: 10.1038/nchembio.1460
Photos, A., Gutierrez, A. and Sommer, R.J. 2006. sem-4/spalt and egl-17/FGF have a conserved role in sex myoblast specification and migration in P. pacificus and C. elegans. Dev. Biol. 293: 142–53. doi: 10.1016/j.ydbio.2006.01.034
Pires-da Silva, A. 2013. Pristionchus pacificus protocols. In: Wormbook (Sommer RJ, ed). The C. elegans Research Community. doi/10.1895/wormbook.1.114.2
Riebesell, M. and Sommer, R.J. 2017. Three-dimensional reconstruction of the pharyngeal gland cells in the predatory nematode Pristionchus pacificus. J. Morph. 278: 1656–66. doi: 10.1002/jmor.20739
Rudel, D., Riebesell, M. and Sommer, R.J. 2005. Gonadogenesis in Pristionchus pacificus and organ evolution: Development, adult morphology and cell-cell interactions in the hermaphrodite gonad. Dev. Biol. 277: 200–21. doi: 10.1016/j.ydbio.2004.09.021
Schlager, B., Wang, X., Braach, G. and Sommer, R.J. 2009. Molecular cloning of a dominant roller mutant and establishment of DNA-mediated transformation in the nematode Pristionchus pacificus. Genesis 47: 300–4. doi: 10.1002/dvg.20499
Serobyan, V., Ragsdale, E.J., Müller, M.R. and Sommer, R.J. 2013. Feeding plasticity in the nematode Pristionchus pacificus is influenced by sex and social context and is linked to developmental speed. Evol. Dev. 15: 161–70. doi: 10.1111/ede.12030
Serobyan, V., Ragsdale, E.J. and Sommer, R.J. 2014. Adaptive value of a predatory mouth-form in a dimorphic nematode. Proc. R. Soc. B Biol. Sci. 281: 20141334. doi: 10.1098/rspb.2014.1334
Sommer, R.J., Carta, L.K., Kim, S.Y. and Sternberg, P.W. 1996. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda, Diplogastridae). Fundam. Appl. Nematol. 19: 511–21. ArticlePDF
Sommer, R.J. and Sternberg, P.W. 1996. Apoptosis and change of competence limit the size of the vulva equivalence group in Pristionchus pacificus: a genetic analysis. Curr. Biol. 6: 52–9. doi: 10.1016/s0960-9822(02)00421-9
Sulston, J.E. and Horvitz, H.R. 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. Article
von Lieven, A.F. 2005. The embryonic moult in diplogastrids (Nematoda) - Homology of developmental stages and heterochrony as a prerequisite for morphological diversity. Zool. Anz. 244: 79–91. doi: 10.1016/j.jcz.2005.05.00
White, J.G., Southgate, E., Thomson, J.N. and Brenner, S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. Roy. Soc. Lond. 314B: 1-340. Article
|