
|
|
|
THE DAUER MUSCLE SYSTEM
 Click pictures for new window with figure and legend, click again for high resolution image Click pictures for new window with figure and legend, click again for high resolution image
1 Anatomy
C. elegans body wall muscle provides animals with the ability to move in sinusoidal waves while lying on their left or right side (see Somatic Muscle). Bodywall muscles are positioned in four quadrants arranged longitudinally along the body’s anterior-posterior axis (DMusFIG 1 & DMusFIG 2; see also MusFIG 10). The two dorsal quadrants are innervated jointly from the dorsal nerve, and the two ventral quadrants from the ventral nerve. This arrangement allows contractions of the dorsal quadrant muscles to be matched with simultaneous relaxations of the ventral quadrant muscles, and vice versa, resulting in smoothly coordinated body movement as waves of muscle contraction proceeding along the length of the body. In the dauer, muscle occupies a relatively larger volume fraction of the bodywall because other tissues are profoundly shrunken. The relative shrinkage of the hypodermis also allows the muscles to occupy a much larger fraction of the outer surface of the bodywall, where they appose to the cuticle.
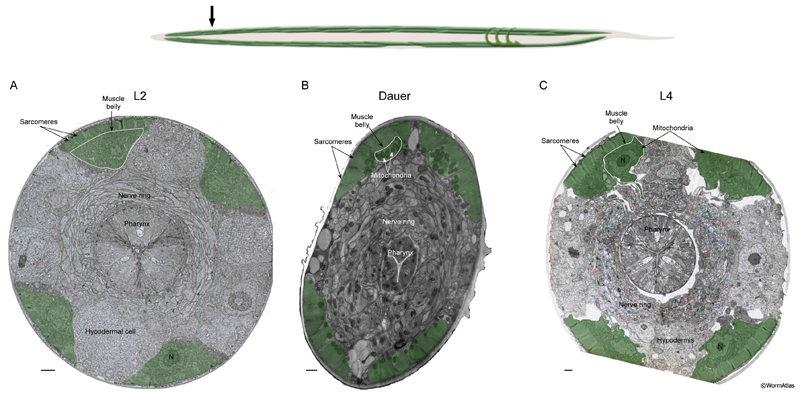
DMusFIG 2: Body wall muscles near anterior intestine. Upper panel, arrow shows approximate position of EM slices in the anterior midbody, near the gonad primordium. A-C. Transverse EM sections showing body wall muscle (green) in an L2, dauer and adult, respectively. In comparison to the L2, the dauer muscle cytoplasm is emptier, containing many large mitochondria. The sarcomeres remain intact in dauers. N, nucleus; DNC, dorsal nerve cord; VNC, ventral nerve cord. Bars, A&B, 1 μm; C, 5 μm. (Image sources: L2 [D. Riddle] L2 28-14 1855; dauer [D. Riddle] 56-1-2 30u; adult [G. Stephney] N506 Z835.)
In the dauer stage, body wall muscles retain well-ordered sarcomeres, which comprise the contractile myofilament apparatus (see MusFIG 9). Compared to the L2 stage, dauer sarcomeres develop into larger structures, with twice as many myosin filaments in the lattice when measuring their depth from the cuticle, and 50% more myosin filaments when measuring side to side from the M-line (DMusFIG 3). The dense body structures linking the myofilaments to the cuticle remain well ordered in dauer muscle and are also larger in proportion to the sarcomere depth. Judging by the increased size of the myofilament lattice, the strength of muscle contraction in the dauer is probably increased by 200% or more compared to the L2 animal, enabling the dauer to respond to stimuli with more powerful motion.
DMusFIG 3: Muscle mitochondria in L2 and dauer larvae. Close-up views of body muscle cells in L2 (A) and dauer (B) larvae. A&B. Lower left, box shows position of enlarged region in original TEM sections; both come from regions near the isthmus of the pharynx. In enlarged views, muscle cells are outlined in green with major components indicated. The dauer muscle belly cytoplasm appears less electron dense than in the L2 muscle and contains many prominent mitochondria, but rather few ribosomes. Lipid storage droplets are also visible in the dauer muscle. Bar, 1 μm. (Image sources: L2 [D. Riddle] L2 28-14 1261; dauer [D. Riddle] 50-2-1 707.)
Although dauer sarcomeres appear enlarged and are functional, the “muscle belly” cytoplasm of dauer muscle is moderately shrunken and filled with relatively large, electron dense mitochondria. Popham and Webster (1979) reported that dauer muscle mitochondria exist in the condensed conformation, which is associated with rate-limited levels of oxidative phosphorylation substrates and reduced respiratory rates (Hackenbrock et al., 1971). Enlarged mitochondria may supply large amounts of energy on a short-term basis to the dauer body muscles as compared to the capabilities of body muscles in L2 larvae.
While the volume fraction of mitochondria in the dauer muscle belly is increased, other cytoplasmic components are reduced (DMusFIG 3). In reproductive animals, the muscle cytoplasm is filled with a granular substance, also known as “ground substance”, representing ribosomal complexes and/or RER as well as other soluble proteins (DMusFIG 3A). In contrast, the dauer cytoplasm appears emptier and less complex, lacking the granular substance in many cases (DMusFIG 3B). Lipid droplets and glycogen storage deposits are also observed more frequently in dauer muscle cells (DMusFIG 4, Popham and Webster, 1979). Muscles are major glycogen reservoirs in nematodes at all stages, and glycogen may be an important energy source for dauers.
DMusFIG 4: Longitudinal view of body wall muscle in dauer and adult animals. Longitudinal view of body wall muscles in a dauer larva and an adult. A. Cartoon showing body wall muscle (green) with box outlining approximate position of EM sections in B-E. B. Enlargement of dauer body wall muscle (outlined in green) showing sarcomeres, mitochondria and lipid droplets. Intestine is outlined in pink. C. Entire TEM section shown in B, with enlarged area outlined. D. Enlargement of adult body wall muscle (green), with intestine outlined in pink. E. Entire TEM section shown in D, with enlarged area outlined. Lipids, yolk droplets and glycogen storage granules are shown. N, nucleus. Bar, 5 μm. (Image sources: Dauer [D. Riddle] 56-6 109; adult [D. Hall] N533 L4 F216.) |
2 Metabolism
Metabolism is slowed in the dauer stage, as evidenced by reduced oxygen consumption and ATP levels (Wadsworth and Riddle, 1988; Vanfleteren and DeVreese, 1996; Houthoofd et al., 2002). In addition, cellular processes are shifted toward energy conservation. For example, the rough ER cisternae and Golgi saccules are smaller in dauers than non-dauers (Popham and Webster, 1979). These metabolic changes allow non-feeding dauers to survive for long periods, relying instead on stockpiles of stored lipids for energy.
Dauer metabolism is specialized for usage of stored lipids, due to relatively reduced activity of the TCA cycle with concomitantly increased glyoxylate cycle (DMusFIG 5; O'Riordan and Burnell, 1990; Wang and Kim, 2003). Fats are stored in the dauer intestine and epidermis (Ogg et al., 1997), converted into glycogen and trehalose and transported to muscles (McElwee et al., 2006). Dauers may also synthesize ATP through anaerobic pathways, such as malate dismutation and ethanol fermentation (Braeckman et al., 2009).
DMusFIG 5: Metabolic differences in L3 and dauer larvae. A. In developing larvae, such as the L3, nutritional intake is distributed from the intestine to target tissues, including muscles (green) and the developing germline (blue). Metabolic resources are utilized through the TCA cycle for growth, development and movement behaviors. B. In dauer larvae, nutritional intake is interrupted by the thick dauer cuticle. During dauer formation, metabolic resources are stored as lipids and glycogen in the intestine and hypodermis. In dauer larvae, these resources are utilized, in part through anaerobic pathways, for locomotion and nictation.
3 Movement Behaviors
Movement behaviors are altered in dauers. While adults and developing larvae actively explore their environment in search of food, dauers tend to lie motionless until physically stimulated (Gems et al., 1998; Gaglia and Kenyon, 2009). This distinctive behavior may help dauers conserve energy. Upon sensing a stimulus, dauers are able to move quite rapidly, somewhat faster than adults. This rapid response to stimuli indicates that the neuromuscular capacity for movement remains intact in dauers, and may even be considered hyper-reactive.
Dauer muscles are highly sensitized to neuronal stimuli. The C. elegans neuromuscular junction is formed by membranous projections, called muscle arms, reaching from the body muscle cytoplasm to the dorsal and ventral nerve cords (see Nervous System: Motor neurons and the motor circuit). In dauers, muscle cells extend greater numbers of these muscle arms to enhance neuromuscular connectivity (Dixon et al., 2008). In addition, dauer muscle demonstrates greater sensitivity to the neurotransmitters that activate muscle movement in C. elegans (Lewis et al., 1987). Efficient and rapid locomotion may help dauers escape from threats and also to target vectors for dispersal to new environments (See Dauer Nervous System: Behavior).
Nictation behavior is specific to the dauer, allowing the animal to wave its body upwards in the air while attached by the tail to a substrate (Cassada and Russell, 1975) (DMusFIG 6; see also Dauer Nervous System Behavior and DBehaviorVID 1 and VID 2). Little is known about the state of muscle contraction during the behavior. Nictation posture may depend on hypercontraction of all muscles and may also be dependent on the rigid dauer cuticle to hold the body straight. Body wall muscle coordination must be altered during the behavior, as the body is held straight with slow, non-propagating body bends.
DMusFIG 6: Dauers performing nictation behavior. C. elegans dauers performing nictation behavior in the wild from the tips of pointed moss leaf tips. In the lower-left of the field, a single dauer is nictating alone. A large mass of dauers nictating together is shown in the center and right regions of the field. (Image source: Marie-Anne Félix, Institute of Biology of École Normale Supérieure.) See also DBehaviorVID 1 and VID 2.
|
4 References
Braeckman, B.P., Houthoofd, K. and Vanfleteren, J.R. 2009. Intermediary metabolism. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.146.1, http://www.wormbook.org. Article
Cassada, R.C. and Russell, R.L. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326-342. Abstract
Dixon, S.J., Alexander, M., Chan, K.K.M and Roy, P.J. 2008. Insulin-like signaling negatively regulates muscle arm extension through DAF-12 in Caenorhabditis elegans. Dev. Biol. 318: 153-161. Article
Gaglia, M.M. and Kenyon, C. 2009. Stimulation of movement in a quiescent, hibternation-like form of C. elegans by dopamine signaling. J. Neurosci. 29: 7302-7314. Article
Gems, D., Sutton, A.J., Sundermeyer, M.L., Albert, P.S., King, K.V., Edgley, M.L., Larsen, P.L., and Riddle, D.L. 1998. Two pleitropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129-155. Article
Hackenbrock, C.R., Rehn, T.G., Weinbach, E.C. and Lemasters, J.J. 1971. Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J. Cell Biol. 51: 123-137. Article
Houthoofd, K., Braeckman, B.P., Lenaerts, I., Brys, K., De Vreese, A., Van Eygen, S. and Vanfleteren, J.R. 2002. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp. Gerontol. 37: 1015-1021. Abstract
Lewis, J.A., Fleming, J.T., McLafferty, S., Murphy, H. and Wu, C. 1987. The levamisole receptor, a cholinergic receptor of the nematode Caenorhabditis elegans. Mol. Pharmacol. 31: 185-193. Abstract
McElwee, J.J., Schuster, E., Blanc, E., Thornton, J. and Gems, D. 2006. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech. Age. Dev. 127: 458-472. Abstract
Ogg, S., Paradis, S., Gottlieb, S., Patterson, G.I., Lee, L., Tissenbaum, H.A. and Ruvkun, G. 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994-999. Abstract
O'Riordan, V.B. and Burnell, A.M. 1990. Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans - II. The glyoxylate cycle and fatty acid oxidation. Comp. Biochem. Physiol. 95B: 125–130. Abstract
Popham, J.D. and Webster, J.M. 1979. Aspects of the fine structure of the dauer larva of the nematode Caenorhabditis elegans. Can. J. Zool. 57: 794-800. Abstract
Vanfleteren, J. R. and De Vreese, A. 1996. Rate of aerobic metabolism and superoxide production rate potential in the nematode Caenorhabditis elegans. J. Exp. Zool. 274: 93-100. Article
Wadsworth, W.G. and Riddle, D.L. 1988. Acidic intracellular pH shift during Caenorhabditis elegans larval development. Proc. Natl. Acad. Sci USA 85: 8435-8438. Article
Wang, J. and Kim, S.K. 2003. Global analysis of dauer gene expression in Caenorhabditis elegans. Development 130: 1621-1634. Article
|

This chapter should be cited as: Wolkow, C.A. and Hall, D.H. 2013. The Dauer Muscle. In WormAtlas. doi:10.3908/wormatlas.XXX
Edited for the web by Laura A. Herndon. Last revision: November 18, 2013. |

|
|
|
|