
|
|
|
THE AGING INTESTINE
 Click pictures for new window with figure and legend, click again for high resolution image Click pictures for new window with figure and legend, click again for high resolution image
1 Structure and Function
Nematodes, such as C. elegans, feed on soil bacteria and other microbes (Schulenburg and Félix, 2017). Food particles are ingested into the alimentary tract by rhythmic pumps of the pharynx in the head where they are concentrated and pulverized (see Hermaphrodite Pharynx). The food particles are pushed from the pharynx into the intestine for digestion and nutrient absorption. The intestine also functions to synthesize and store macromolecules, initiate immune responses, and nurture germ cells by producing and secreting yolk (Kimble and Sharrock, 1983; Schulenburg et al., 2004; Pauli et al., 2006; McGhee, 2007; Hermaphrodite Intestine).
In C. elegans, the intestine is comprised of 20 large epithelial cells that are mostly positioned as bilaterally symmetric pairs to form a long tube with a central lumen (Avery and Thomas, 1997). Intestinal cells are large, cuboidal, and bound tightly to each other by adherens junctions on their apical sides and gap junctions and septate-like junctions on their lateral sides (AIntFIG 1). Each intestinal cell forms part of the lumen at its apical pole and secretes components of the basal lamina from its basal pole. The microvilli, composed of an actin filament core anchored by a web of intermediate filaments in the terminal web, protrude to form the brush border at the intestinal cell apical membranes (AIntFIG 1). A glycocalyx of highly modified glycoproteins coats the microvilli. Possible functions for the glycocalyx include digestive enzyme localization, protection from physical or toxic injury or filtration of absorbed components (Lehane, 1997).
During development, two intestinal primordial cells gastrulate, divide, polarize, and intercalate, to form the 20 adult intestinal cells (for details of this process see Hermaphrodite Intestinal Development). Some intestinal cells become binucleate, forming a total of 30-34 intestinal nuclei. These nuclei endoreduplicate during each larval molt resulting in visibly larger nuclei with prominent nucleoli in adulthood (Sulston and Horvitz, 1977; Hedgecock and White, 1985; Leung et al., 1999). Intestinal cells also contain, many mitochondria, extensive rough endoplasmic reticulum, many ribosomes and an extensive collection of membrane-bound vesicles and vacuoles (for more details see Hermaphrodite Intestine Structure and Function).
AIntFIG 1: Structure of young adult intestine. A. The intestine is positioned on the left side of the body anterior to the vulva and on the right side of the body posterior to it. At its anterior end, the intestine is connected to the pharynx via the pharyngeal valve. The most posterior portion is squeezed by the stomatointestinal muscle (not shown), near where the intestine connects to the rectum and anus. Arrowhead indicates the position of the TEM cross-section shown in C. (Adapted with permission from Mendenhall, 2015.) B. Key structural elements of the healthy intestinal cytoskeleton. At its basal pole the intestine is covered by a basal lamina (orange), separating it from the pseudocoelom. Pairs of intestinal cells meet to form a lumen between them, with the two cells firmly linked by adherens junctions at their apical borders. Gap junctions and smooth septate-like junctions form a complex junction just beneath the adherens junctions on the lateral membranes where the two intestinal cells meet. Intermediate filaments help to anchor a terminal web of fibers running just beneath the microvilli that face the lumen itself. An actin-based cytoskeleton fills each villus; the actin fibrils anchor into the terminal web at one end, and to an electron dense cap at the tip of the villus. A thick glycocalyx covers the outer surface of the microvilli. At adulthood, most intestinal cells contain two very large nuclei (black circle). The lumen of the young adult intestine usually is filled by debris from partially digested bacteria, but few if any intact bacteria. Graphic adapted from Wood et al. (1996). C. Electron micrograph showing the key features of the third ring of the young adult intestine. The intestinal cytoplasm is filled with a complex mixture of organelles, including mitochondria, Golgi apparatus, RER, yolk-filled granules, and occasional large autophagosomes. A complex junction (arrow) next to an adherens junction (arrowhead) seals the two intestinal cells to each other (inset). A basal lamina covers the surface of the intestine facing the pseudocoelom. (Image source: N510 [Hall] G127.) D. Electron micrograph showing details of the junctions connecting two intestinal cells. AJ, adherens junction; GJ, gap junction; SSJ, smooth septate junction. (Image source: N501B [Hall] 8004.)
2 Changes in the Intestine with Age
2.1 Overview of Structural Changes
Progressive degradation of the intestine includes loss of intestinal microvilli and nuclei as well as changes in the size, shape and cytoplasmic contents of the organ (McGee et al., 2011; Herndon et al., 2017).
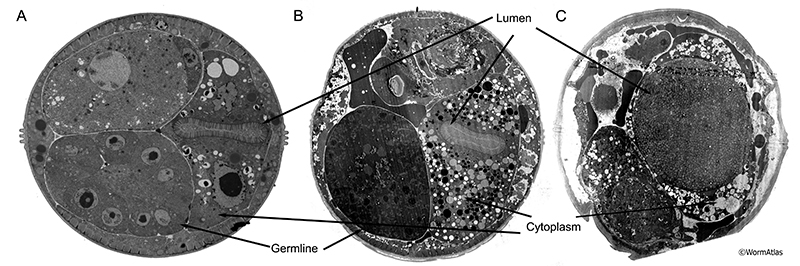
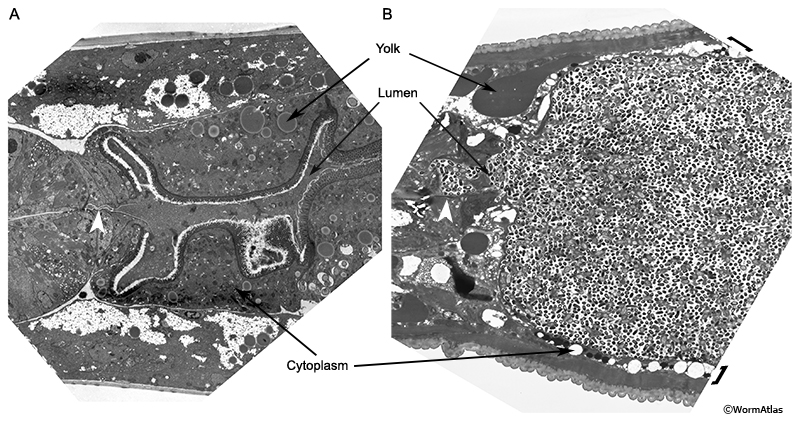
During late larval development, the expanding reproductive system pushes the intestine to one side of the body cavity (Sulston and Horvitz, 1977; Kimble and Hirsh, 1979). In young adults, the intestinal lumen is nearly uniform in size and shape along its entire length. Throughout adulthood, the germline progressively swells (Golden et al., 2007) creating pressure on intestine strong enough to cause severe compression of the intestinal epithelium, particularly in the midbody (AIntFIG 5). In older adults, the intestinal lumen may become thinner, winding, or swollen in localized regions (AIntFIG 2; AIntFIG 3). Undigested bacteria often fill the lumen and microvilli become shorter and sparser (AIntFIG 2; AIntFIG 3; AIntFIG 6). Dramatic cytoplasmic changes occur with phenotypes ranging from accumulation of lipids, yolk and autophagic material (AIntFIG 3; AIntFIG 7), to significant lysis of components (AIntFIG 7) and in many cases, loss of intestinal nuclei has been noted (AIntFIG 8; AIntFIG 9).
AIntVID 1&2: Videos of cross-sections of young and old worms. Videos are created from aligned methylene blue/paraosaniline cross sections and progress from the nose to tail of the animals. The intestine and other basic anatomical structures are labeled. AIntVID 1 is from a 1-day-old adult wild-type worm. Intestine is visible from 5-45 seconds. From 20-25 seconds, the intestine becomes visibly squished to a smaller volume as the enlarged uterus fills much of the interior cavity. While the tissue appears to spiral around due to an alignment artifact, it actually lies toward the dorsal pole in this region near the uterus. The intestinal cytoplasm features many large vacuoles in this specimen, with the lumen staying quite narrow except as seen during the sections from 6-7 seconds. AIntVID 2 is from a 17-day-old wild type adult worm with the intestine visible between 5-53 seconds. The intestine becomes visibly squished to a much smaller volume by the presence of a large germline tumor at two points along the length of the animals, shown from 21-26 seconds and again between 38-40 seconds and even seems to vanish in the most restricted portions. The cytoplasm in this aging animal is much less stained, appearing almost white due to the loss of ground substance and the expanding sizes and loss of content inside the vacuoles. The lumen is swollen and darkly stained by its bacterial contents at 5-6 seconds. As in AIntVID 1, at points the intestine appears to spiral, but this effect is an artifact of the alignment of the video frames. (Video Source: McGee et al., 2011.)
2.2 Accumulation of Bacteria
Pockets of undigested bacteria are often visible in the pharyngeal and intestinal lumens in aging adults. These bacteria are likely undigested due to reduced pumping and grinding efficiency in these older animals (Collins et al., 2008; McGee et al., 2011). In rare cases, intact bacteria have been observed invading the lumen of the uterus and spermatheca (entering via the vulva), or crossing into cells along the alimentary canal by infecting the marginal cells of the pharynx (McGee et al., 2011). In older animals, bacteria often begin to divide inside the nematode body, distending the intestinal lumen and compressing other tissues against the body wall. In some portions of the intestinal lumen, bacteria are found closely opposed to microvilli and may actively damage them (see Degradation of Intestinal Lumen and AIntFIG 6). Surprisingly, bacteria have not been observed inside the cytoplasm of intestinal cells of old adults by electron microscopy, although these animals could have been inadvertently excluded from analysis due to rapid death after this event.
 |
2.3 Accumulation of Yolk
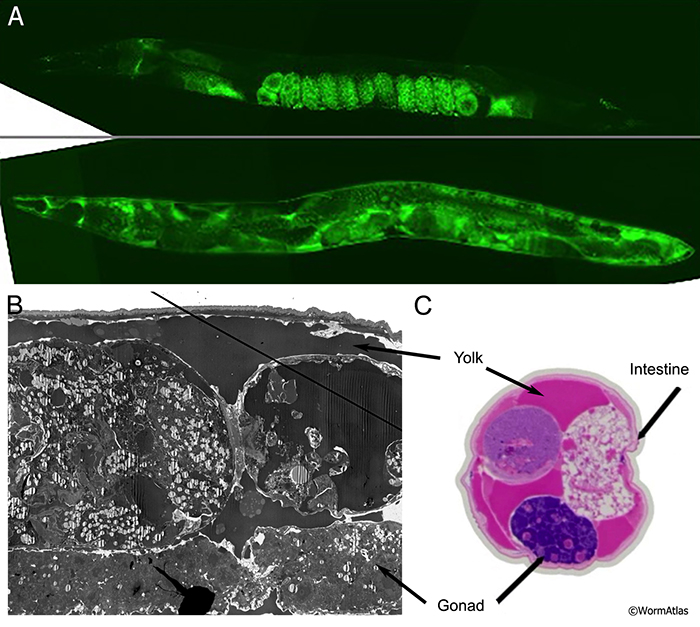
In young adults intestinal cells synthesize yolk particles that are secreted into the body cavity and then are taken up by oocytes in the gonad (Kimble and Sharrock, 1983; Hall et al., 1999). The yolk particles provide nourishment to developing embryos (PeriFIG 2C) (Kimble and Sharrock, 1983; Sharrock, 1983; Hall et al., 1999) (see also Hermaphrodite Intestine Structure and Function). However, in post-reproductive animals that lack a functional germline, the intestine continues to produce yolk which accumulates in the body cavity (AIntFIG 4) (Garigan et al., 2002; Herndon et al., 2002; McGee et al., 2011). Accumulated yolk has been identified by using GFP (AIntFIG 4A) (Garigan et al., 2002; Herndon et al., 2002), transmission electron microscopy (AIntFIG 4B) (Herndon et al., 2002; McGee et al., 2011) and methylene blue/parasosaniline staining (AIntFIG 4C; AIntVID 2) (McGee et al., 2011). In thin section as seen by TEM, it becomes obvious that rather little of this excess yolk remains inside the intestinal cell cytoplasm. Indeed the relative amount of yolk storage in the cytoplasm is already dropping in the younger adults day by day (compare AIntFIG 2A and AIntFIG 3A to AIntFIG 2B). The full effects of this yolk accumulation in the pseudocoelom are not currently known, but it appears that it is detrimental and limits lifespan of C. elegans (Gems and de la Guardia, 2013).
2.4 Degradation of Intestinal Lumen and Microvilli
In young animals, the intestinal lumen appears uniform in its size and shape throughout the entire length of the intestine, with the exception of a localized swelling near the pharynx. In contrast, old intestinal lumens appear thinner, tortuous, and sometimes swell locally (AIntFIG 5; AIntVID 2) (McGee et al., 2011). In regions with very swollen germline , the intestine is compressed against the side of the worm into a narrow wedge(AIntFIG 5A) (Golden et al., 2007).
AIntFIG 5: Compression of the intestine during aging.
A. Transverse cross-section of 15-day-old (class B) nematode showing extreme compression of the intestine between the gonad and the body wall, such that the cytoplasm consists of a faint narrow electron dense stripe surrounding the lighter lumen. No evidence of cytoplasmic organelles can be detected here. (Image source: N816 [Hall] H0027.) B. Longitudinal section of 15-day-old (class B) adult nematode showing some compression of the intestine and degradation of the cytoplasm of the intestine. There are many vacuoles and lighter-staining vesicles filling the cytoplasm, but rather little ground substance surrounding them. The dark circular object along the right edge of the intestine is not a nucleus, but a fragment of distal germline poking into the intestine. (Image source: N815 [Hall] G702.)
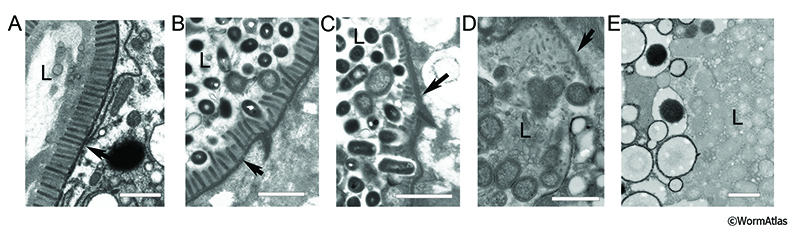
Similarly, microvilli within the young adult intestine appear to have a consistent anatomy from cell to cell, except for those within the first and last rings of cells, INT1 and INT9, where villi are moderately foreshortened. Analysis of older animals in various states of decline have indicated that the most decrepit of the aging animals tend to show significant microvilli degeneration with shortening, and in some cases a complete loss, of the microvilli that normally face the lumen. However, these microvillar defects can vary from cell to cell within the same animal. In some cases, healthy microvilli are found in areas with significant intestinal distortion and near other cells that show severe microvilli phenotypes (AIntFIG 6) (McGee et al., 2011). Conversely, some relatively healthy looking animals show shortened villi phenotypes in their intestinal cells, reflecting the mosaic pattern of cell decline among intestinal cells in a given animal, or in comparison to the rate of decline in other tissues (Herndon et al., 2002; Herndon et al., 2017). In some areas, individual bacteria attach firmly to the base of the villi, and may even appear embedded into the villi in a manner suggesting that they may cause local damage (AIntFIG 6B&C).
|
2.5 Changes in the Cytoplasm
In addition to changes within the intestinal lumen, there are also dramatic age-related changes in the cytoplasm of intestinal cells. In older animals, some intestinal cells contain abundant lipid droplets or vacuoles not found in cells of younger animals animals (compare AIntFIG 2A and AIntFIG 3A to AIntFIG 7B) (Herndon et al., 2002; McGee et al., 2011). The detailed contents vary rather dramatically among older adult animals, even when they are the same age. While some animals show completely different organelles within the cytoplasm (AIntFIG 7A&B), in other instances cells can appear to have a lytic cytoplasm or have reduced cytoplasmic volume (AIntFIG 7C) and in rare cases, individual intestinal cells appear to become reduced in cytoplasmic volume compared to their neighbors, suggesting that autophagy may progressively degrade other components besides the nucleus (McGee et al., 2011). Individual cells within the same animal show variations in cytoplasmic details further suggesting a stochastic nature of to these morphological changes that accompany aging. These regional variations in the intestine of older animals, therefore, may be governed by local processes, perhaps by cell-specific nuclear changes (see section 2.6 Loss of Intestinal Nuclei), or even by the effects of bacterial attack. Indeed these varying intestinal phenotypes are not unlike separate “disease states”, even though all animals were wild type.
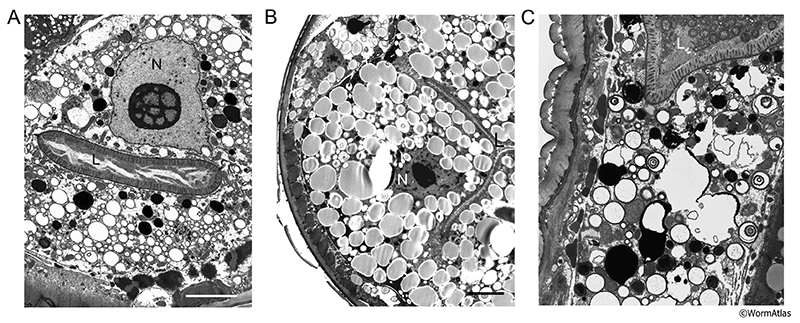
2.6 Loss of Intestinal Nuclei
Intestinal cells also show a progressive decline in the integrity of their nuclei with age. Nucleoli often show dramatic enlargement by TEM (AIntFIG 7A). In some cases, individual nuclei appear shrunken with darker staining, or possibly undergo autophagy (AIntFIG 7B and AIntFIG 9) (McGee et al., 2011). In young adults most intestinal cells have two nuclei, so that the 20 adult cells contain about 30-32 large nuclei in total. As the animals age, some intestinal nuclei appear visibly smaller and in decrepit nematodes, many intestinal nuclei are undetectable by DAPI staining or by visualizing stained tissue sections, so that some cells seem to have no remaining nucleus (AIntFIG 8) (McGee et al., 2011). This degradation and loss of nuclei occurs throughout the intestine, but seems most severe in the anterior half of the worm. This stochastic loss of nuclei with age represents another example of increased variability with age. The loss of nuclear function surely results in dramatic reduction in gene expression and cell homeostasis in the intestine, with knock-on effects on the health of the whole animal (Pincus and Slack, 2010).

AIntFIG 9: Nuclear degeneration in aging intestinal cells. A. A 7-day-old animal in the midbody, where the intestine has been pushed into a thin dorsal wedge by swelling of the uterus. Two intestinal nuclei are seen, a full sized nucleus on the right, with an enlarged nucleolus, and a shrunken dark-staining nucleus undergoing phagocytosis on the left, where the swollen nucleolus virtually occupies the whole volume. Inset Enlarged view of A. Arrows indicate internal membranes enveloping the shrunken nucleus. N, nucleus.
B. A nucleus from a 15-day-old animal is reduced in size and undergoing phagocytosis. Here again there is a suggestion of internal membranes beginning to envelop the shrinking nucleus, which is marked by clumping chromatin. Scale bars indicate 0.5 micron.
3 References
Avery D.G. and Thomas, J.H. 1997. Feeding and defecation. In C. elegans Volume II. Ed.s Riddle D.L., Blumenthal, T., Meyer B.J. and Priess J.R . Pp 679-716. Cold Spring Harbor Laboratory Press. Article
Collins, J.J., Huang, C., Hughes, S. and Kornfeld, K. 2008. The measurement and analysis of age-related changes in Caenorhabditis elegans.WormBook. ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.137.1 Article
Garigan, D., Hsu, A.L., Fraser, A.G., Kamath, R.S., Ahringer, J. and Kenyon, C. 2002. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161:1101–1112. Article
Gems, D. and de la Guardia, Y. 2013. Alternative perspectives on Aging in Caenorhabditis elegans: reactive oxygen species or hyperfunction? Antioxid. Redox Sig. 19: 321-29. Article
Golden, T.R., Beckman, K.B., Lee, A.H.J., Dudek, N., Hubbard, A., Samper, E. and Melov, S. 2007. Dramatic age-related changes in nuclear and genome copy number in the nematode Caenorhabditis elegans. Aging Cell 6:179–188. Article
Hall, D.H., Winfrey, V.P., Blauer, G., Hoffman, L., Furuta, T., Rose, K.L., Hobert, O. and Greenstein, D. 1999. Ultrastructural features of the adult hermaphrodite gonad of C. elegans: relations between the germ line and soma. Dev. Biol. 212: 101-123. Article
Hedgecock, E.M. and White, J.G. 1985. Polyploid tissues in the nematode Caenorhabditis elegans. Dev. Biol. 107: 128–133. Abstract
Herndon, L.A., Schmeissner, P.J., Dudaronek, J.M., Brown, P.A., Listner, K.M., Sakano, Y., Paupard, M.C., Hall, D.H. and Driscoll, M. 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419: 808-814. Article
Herndon, L.A., Wolkow, C.A., Driscoll, M. and Hall, D.H. 2017. Effects of ageing on the basic biology and anatomy of C. elegans. InAgeing: lessons from C. elegans. (ed Olsen, A. and Gill, M.). Chapter 2. pp. 9-39. Springer International, Switzerland. Abstract
Kimble, J. and Hirsh, D. 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396-417. Article
Kimble, J. and Sharrock, W. J. 1983. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev. Biol. 9: 189-196. Abstract
Lehane, M.J. 1997. Peritrophic matrix structure and function. Annu. Rev. Entomol. 42: 525–550. Abstract
Leung, B., Hermann, G.J. and Priess, J.R. 1999. Organogenesis of the Caenorhabditis elegans intestine. Dev. Biol. 216: 114–134. Article
McGee, M.D., Weber, D., Day, N., Vitelli, C., Crippen, D., Herndon, L.A., Hall, D.H. and Melov, S. 2011. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 10:699-710. Article
McGhee J.D. 2007. The C. elegans intestine. In WormBook (ed. The C. elegans Research Community), WormBook, doi/10.1895/wormbook.1.133.1. Article
Mendenhall, A.R., Patricia M. Tedesco, P.M., Sands, B., Johnson, T.E. and Brent, R. 2015. Singlecell quantification of reporter geneexpression in live adult Caenorhabditis elegans reveals reproducible cell-specific expression patterns and underlying biological variation. PLoS ONE. 10: e0124289. Article
Pauli, F., Liu, Y., Kim, Y.A., Chen, P.-J. and Kim, S.K. 2006. Chromosomal clustering and GATA transcriptional regulation of intestine-expressed genes in C. elegans. Development 133: 287-295. Article
Pincus, Z. and Slack, F.J. 2010. Developmental biomarkers of aging in Caenorhabditis elegans. Dev. Dynam. 239: 1306-14. Article
Schulenburg, H. and Félix, M-A. 2017. The natural biotic environment of Caenorhabditis elegans. Genetics 206: 55-86. Article
Schulenburg, H., Kurz, C.L. and Ewbank, J.J. 2004. Evolution of the innate immune system: the worm. Immunol. Rev. 198: 36-58. Abstract
Sharrock, W.J. 1983. Yolk proteins of C. elegans. Dev. Biol. 96: 182-188. Abstract
Sulston, J.E. and Horvitz, H.R. 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. Article
Wood, W.B., Bergmann, D. and Florance, A. 1996. Maternal effect of low temperature on handedness determination in C. elegans embryos. Dev. Genet. 19:222–230. Abstract
|

This chapter should be cited as: Herndon, L.A., Wolkow, C.A. and Hall, D.H. 2018. The aging intestine. In WormAtlas.
Edited for the web by Laura A. Herndon. Last revision: May 14, 2018. |

|
|
|
|