
Factors That Determine Connectivity in the Nervous System of Caenorhabditis elegans
J.G. White, E. Southgate, J.N. Thomson, et al.
Laboratory of Molecular Biology, Medical Research Council Centre, Hills road, Cambridge, CB2 2QH, England
General Features of the Nervous System of C. elegans - Neighborhoods and Synaptic Partners - The Neighborhoods of AIA and AIB -
Synaptic Variability Between Animals - Similar Neurons in Different Neighborhoods - Summary - Discussion - Acknowledgment - References
A nervous system is an ensemble of interconnected cells (neurons), the function of which is to transduce, transmit, and process information. Information is encoded within the nervous system as membrane potentials and processed at synaptic junctions between neurons. The information-processing capabilities of a nervous system are determined by the properties of the component neurons and their synaptic junctions together with the pattern of interconnections between them. At the present time, a considerable body of information has been accumulated on synaptic transmission and its relatinship to the membrane potential, but little is known about the mechanisms by which specific patterns of interconnection are establised and maintained between neurons. A typical vertebrate nervous system may contain upwards of 10¹º neurons (Jacobson 1978), each having many synaptic connections; such vast complexity obviously poses severe difficulties for the study of synaptic specificity in such animals. Some invertabrates, however, have nervous systems that have only a few hundred cells and are also sufficiently small that the detailed pattern of synaptic connections that exist between all the component neurons may be deduced at an ultrastructural level. The soil nematode Caenorhabditis elegans is such an animal; it has a total complement of 302 neurons, and the structure of the entire nervous system has been ascertained by reconstructions of electron micrographs of serial sections (Ward et al. 1975; Ware et al. 1975; Albertson and Thomson 1976; White et al. 1976; J.G. White et al., in prep.). A knowledge of the complete structure does not in itself reveal the underlying mechanisms that were used in the generation of the structure, but at least it allows one to focus on a well-defined set of interconnected neurons, and to ask specific questions concerning the establishment of the pattern of synaptic contacts that are present. In this paper, we would like to consider the problem of synaptic specificity in the context of the nervous system of C. elegans. In particular, we would like to discuss some of the factors that may determine the set of synaptic contacts made by a given neuron.
General Features of the Nervous System of C. elegans
The 302 neurons that make up the nervous system of C. elegans have been put into 118 classes (J.G. White et al., in prep.). The classes were defined on the basis of morphology, ultrastructure, and patterns of synaptic contact; the largest class has 13 members each with the same morphology and connectivity, but many classes have only a single, unique member. Thus, although C. elegans has only a small number of neurons in its nervous system, it nevertheless has a rich variety of cell types. Individual neurons generally have a simple, relatively unbranched structure; in fact, many are simple monopolar cells with unbranched processes. Neuron processes run in spatially coherent parallel process bundles making en passant synapses to neighboring processes. Process bundles generally run alongside ridges of hypodermis and are bounded by a basal lamina. They run either longitudinally or circumferentially. The circumpharyngeal nerve ring is, as its name implies, a circular bundle of processes that surrounds the pharynx. It is the most extensive region of neuropil in the animal and is where most of the sensory integration takes place. A large, longitudinal, posteriorly directed bundle of processes leaves the nerve ring ventrally and runs along the length of the animal along the ventral midline. This ventral nerve cord is made up mainly of the processes of motor neurons that innervate body muscles and their associated interneurons. Motor neuron axons do not approach muscle cells but stay confined to process bundles. They run adjacent to the surface of bundles and synapse through the bounding basal lamina onto muscle arms, which are long, neuronlike processes that emanate from muscle cells.
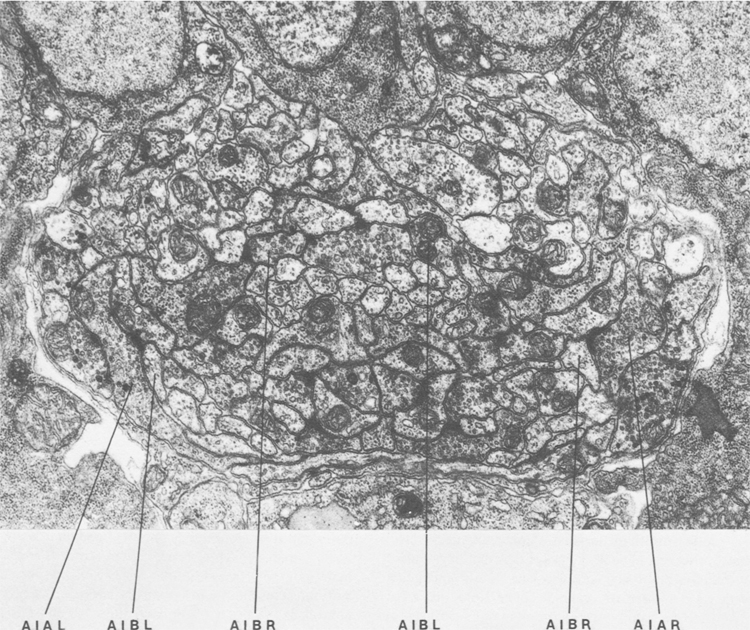
Figure 1. Cross section of the anterior ventral cord near the junction with the nerve ring. The proximal regions of processes of AIB run in close association the processes of AIA near the lateral amrgins of the cord. The distal regions of AIB run in the interior of the process bundle in different neighborhoods. Magnification, 28,000x.
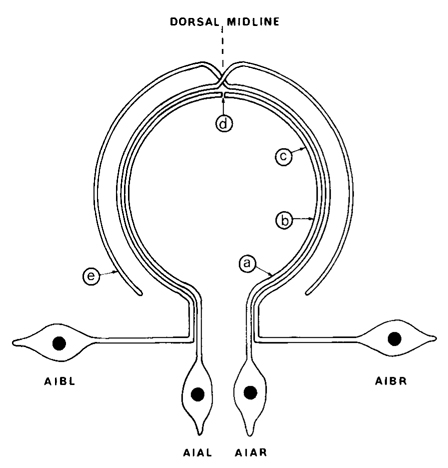
Figure 2. Diagram showing the arrangement of the processes of AIA and AIB in the ventral cord and nerve ring. The nerve ring runs circumferentially round the pharynx and the ventral cord runs longitudinally, but the two regions have been drawn in the same plane for simplicity. AIA and AIB are two classes of interneurons; each with two bilaterally symmetric members. AIA has cell bodies situated ventrally that send processes into the ventral cord. These then enter the nerve ring, run round it, and eventually meet and terminate on the dorsal midline. AIB has cell bodies situated laterally that have procsses that enter the ventral cord via commissures. These then enter the nerve ring and run around it, in close association with the processes of AIA. When the processes of AIB reach the dorsal midline, they make an abrupt change of neighborhood and then continue around the nerve ring, eventually terminating ventrally. The labels a-e refer to the panels of Fig.3.
Neighborhoods and Synaptic Partners
As a consequence of the organization of the nervous system of C. elegans into parallel bundles of unbranched processes, a given process is adjacent to only a few other processes at any point. Furthermore, process bundles are spatially coherent, i.e., processes tend to maintain their relative positions within the bundle; thus, the set of neighboring processes tends to remain constant over the length of a process. Figure 1 shows a cross section of the ventral nerve cord near the junction with the nerve ring. The cord is bilaterally symmetrical at this point, and, as can be seen, processes such as those of AIA and AIB run in nearly identical configurations on each side of the cord. We define the set of processes that are adjacent to the processes of a given neuron as its neighborhood. Since synaptic contacts are made between adjacent processes, then the set of synaptic partners of a neuron has therefore to be a subset of its neighborhood. In the course of determining the structure of the nervous system of C. elegans, three nerve rings were reconstructed. These were designated the T, U, and H series reconstructions. On one of these (H), we undertook a complete neighborhood analysis on one representative of each neuron class that had processes in the nerve ring. The degree of adjacency of each neighbor was determined by counting the number of sections in which it was adjacent to the process of the neuron under consideration. The numbers thus obtained were normalized to the neighbor with the greatest adjacency. The data thus obtained were plotted in tabular form along with the synaptic data. We have selected a few examples of neuron classes that illustrate most of the features that were seen.
The Neighborhoods of AIA and AIB
AIA and AIB are two classes of interneurons, each with two bilaterally symmetrical members designated AIAL, AIAR, AIBL, and AIBR (classes have been given three-letter names that may, in addition, have symmetry descriptions appended if there are dorsal and ventral [D,V] and/or left and right [L,R] members of the class). The AIA cell bodies are situated in the ventral ganglion and send processes into the anterior regions of the ventral cord (Fig.1). These processes then enter the nerve ring ventrally and run around each side of it, meeting and terminating at the dorsal midline (Fig.2). Figure 3 shows a set of micrographs of the neighborhood of AIAR taken at various points in the nerve ring. The neighborhood and synapses of AIAR are shown in Table 1. AIAR has a total of 13 neighbors and, surprisingly, has synaptic contacts (i.e., is postsynaptic, presynaptic, or has gap junctions) with many of them. The process of AIAR is closely associated with those of AIMR, AIBR, ASGR, and ASKR; it has rather loose associations with several others. The close association of a small group of processes that have a rather looser association with the rest of their neighborhood is a common feature of the process bundles of C. elegans.
The AIB cell bodies are situated laterally and send processes into the ventral cord. These processes then enter the ring ventrally and run around each side of it in close association with the processes of AIA (Fig.2). The processes of AIA terminate on the dorsal midline, but when the processes of AIB reach this point they abruptly change direction and run for a short distance across the process bundle. They then revert to their former trajectory around the nerve ring but are now in a completely different neighborhood where that run in close association witht the processes of a motor neuron, RIM (Fig.3e), eventually terminating in the ventral region of the nerve ring. Thus, the distal regions of the processes of AIB run in a different neighborhood to the proximal, and, as a consequence, the synaptic contacts made by the distal and the proximal regions are completely different (Table 2). Another probable consequence of the extended neighborhood of AIB is that it has significantly more synaptic partners than AIA; indeed, AIB has as many synaptic partners as AIA has neighbors.
The observation that the neurons of C. elegans inhabit restricted neighborhoods, yet are generally highly locally connected within their neighborhoods, suggests that a neuron of a given class has the potential to form synaptic connections to many different classes yet is only able to make connections to those classes that are locally accesible. Such a notion predicts that if it were possible to transplant a neuron to a different neighborhood, then it would form synaptic contacts with cell classes that are never normally contacted. Such an experiment is not at the moment feasible in C. elegans, but there is some further circumstantial evidence that supports such an idea.
Table 1. AIAR-Neighborhoods and synapses
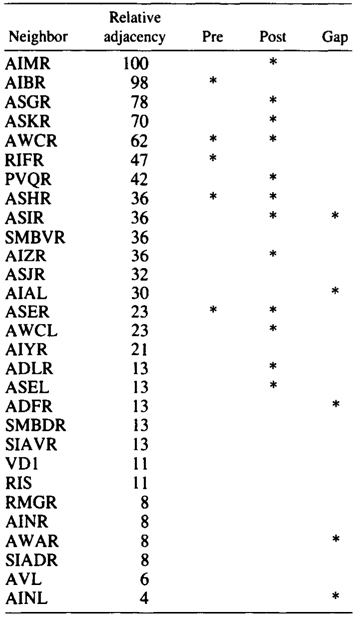
Table shows normalized relative adjacencies of all the neighbors
of AIAR together with the three types of synaptic contacts (*),
i.e., presynaptic, postsynaptic, and gap junctions.
Synaptic Variability between Animals
The three nerve rings that were reconstructed had essentially the same structure and connectivity. There was, however, some low-level variability with respect to the number of synapses seen between classes that were connected synaptically and also some qualitative variation in that some pairs of neuron classes had synaptic contacts in one animal that were not present in another. All the animals reconstructed had the same genotype, so there could not be any genetic basis for this variation. As a neighborhood analysis was done on all the neurons in the nerve ring of the H series, it was possible to see which of the class pairs that had synaptic connections in the U series, but not in the H series, were neighbors in the H series. There were 39 cases where there were two or more unambiguous synapses between particular classes of neurons in the U series that were not present on the H series. Of these 39 cases, 15 involved class pairs that were not neighbors in the H series and therefore could not make synaptic contacts. An example is illustrated in Figure 4, where CEPVR synapses onto SIAVR in the U series. The relative position of the process of CEPVR in the H series is displaced to the right compared with that in the U series and never runs adjacent to the process of SIAVR. Thus, many of the qualitative differences in synaptic connectivity seen between animals may arise because of slight differences in the relative location of the processes involved within the process bundles. Similar variations in synaptic connections arising from variations in structure have also been seen on a locust interneuron (Pearson and Goodman 1979).
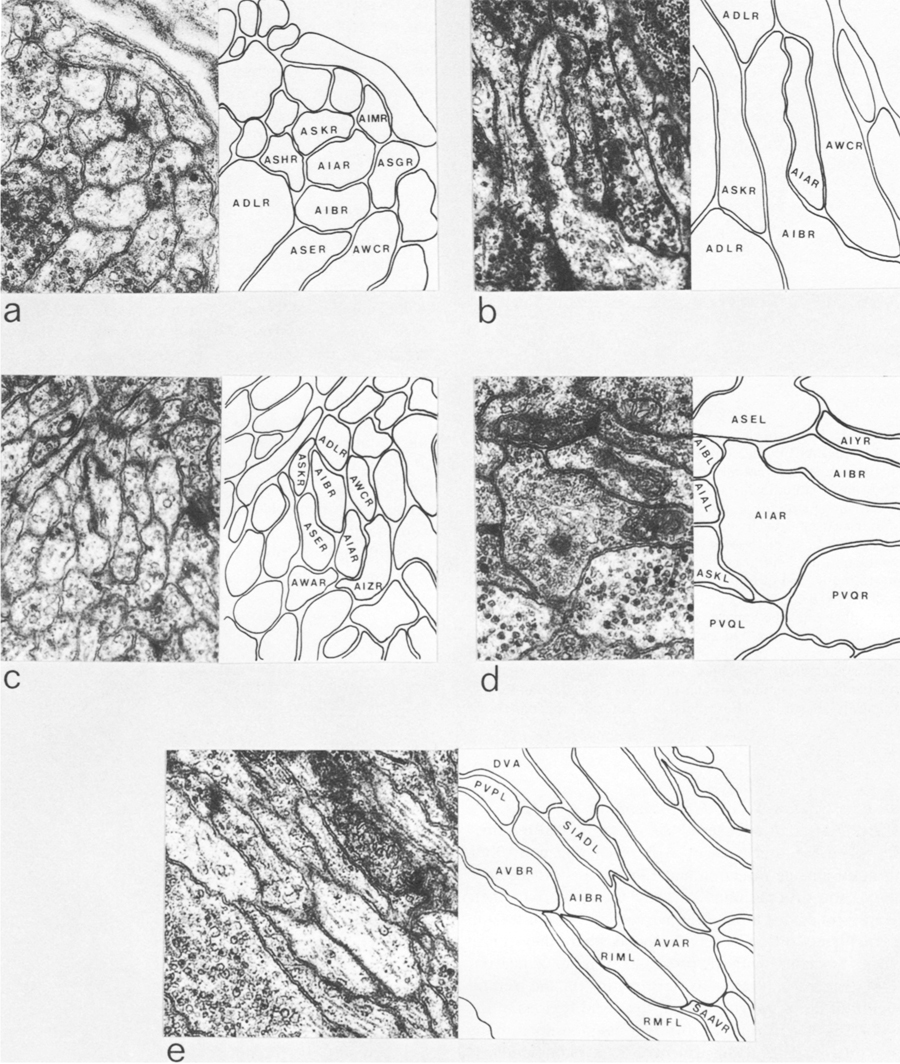
Figure 3. Set of electron micrographs with associated tracings showing the neighborhoods of AIA and AIB in the nerve ring. Fig.2 shows the location of each panel in the ring. Process of AIB are closely associated with those of AIA proximally (a-d) but move to a different neighborhood distally where they are closely associated with the process of RIM (e). Magnification, 30,000x.
Table 2. AIBR-Neighborhoods and synapses
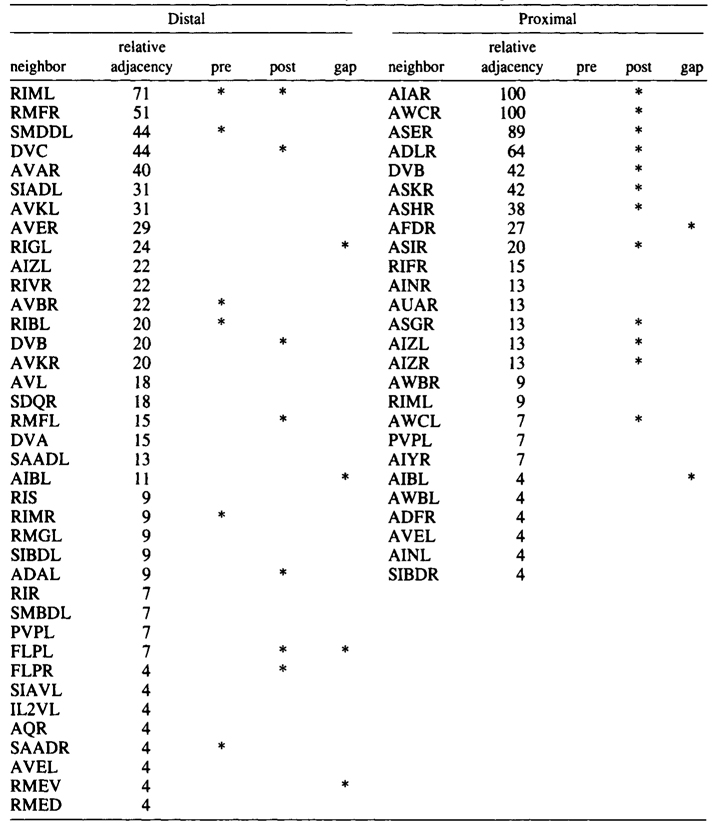
Table shows normalized relative adjacencies of all the neighbors of AIBR together with the three types of synaptic contacts (*),
i.e., presynaptic, postsynaptic, and gap junctions.
Similar Neurons in Different Neighborhoods
AQR and PQR are two neurons that are similar in many respects and probably belong to the same class, although they have been given different class names. Both have a small cilium that is not part of a sensillum, and they are derived from equivalent positions in two bilaterally symmetric lineages (Sulston and Horvitz 1977; Sulston et al. 1983). During development, AQR migrates to the head of the animal and sends a process into the nerve ring. PQR migrates toward the tail and sends a process into the posterior end of the ventral cord. Their processes therefore inhabit different neighborhoods. There are several differences in their sets of synaptic partners, which is the reason why they were originally assigned to seperate classes. The neighborhoods and synapses of AQR and PQR are shown in Tables 3 and 4. There are seven neighbors that are common to AQR and PQR, and similar patterns of synaptic contact are made by both AQR and PQR to five of these, suggesting again that AQR and PQR might really be members of a single class. The main differences in synaptic connectivity between AQR and PQR arise from connections that are made to classes of neurons that are unique to one neighborhood or the other. PQR has three synaptic partners that are not available to AQR, and AQR has five that are not available to PQR. Thus, perhaps PQR could be though of as AQR transplanted from the head into the tail, and likewise the converse for AQR. Again the implication is that neurons have the potential to form more classes of synaptic connection than they actually do.
Table 3. AQR-Neighborhoods and synapses
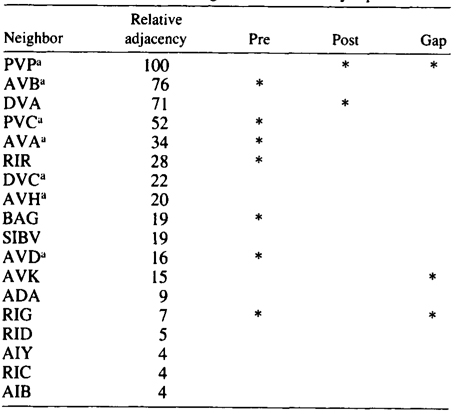
Table shows normalized relative adjacencies of all the neighbors of AQR together with the three types of synaptic contacts (*),
i.e., presynaptic, postsynaptic, and gap junctions.
a-Neighbors in common with PQR
Table 4. PQR-Neighborhoods and synapses
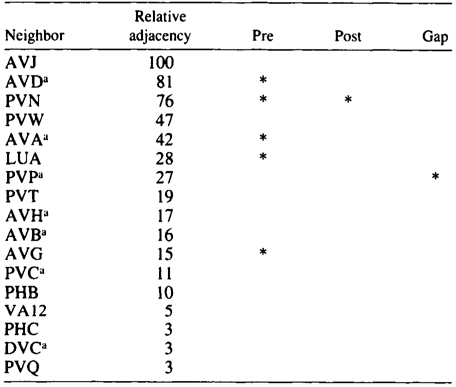
Table shows normalized relative adjacencies of all the neighbors of AQR together with
the three types of synaptic contacts (*), i.e., presynaptic, postsynaptic, and gap junctions.
a-Neighbors in common with AQR
Summary
The nervous system of C. elegans is arranged as a collection of process bundles. Processes within bundles are generally unbranched and occupy defined positions relative to the neighbors. Small groups of processes are often closely associated together and run adjacent to one another for relatively long distances. We have defined the set of neurons that have processes adjacent to the processes of a given neuron as a neighborhood of that neuron. Synapses in C. elegans are made en passant between adjacent processes. Of the 1165 pairs of adjacent processes that were analyzed, 520 (45%) had synaptic contacts. The set of neurons that make synaptic contact with a given neuron is therefore, on average, 45% of that neurons neighborhood. Neurons make synaptic contacts with fewer classes of partners than they have the potential for, as they are limited in their choice of partner to those that inhabit their neighborhood. Some classes of neurons have processes that make abrupt transitions from one neighborhood to another. There is usually some identifiable cue at the transition point, such as the termination of a closely associated process or a discontinuity at the junction of one process bundle with another. Neurons that inhabit more than one neighborhood have a more extended set of synaptic partners than those that are confined to a single neighborhood.
Discussion
We have proposed, and presented some circumstantial evidence in support of, the notion that neurons in C. elegans have the potential for making many more classes of synaptic connection than they actually do and that the placement of the processes of neurons into particular neighborhoods is a major factor in determining synaptic connectivity. This, of course, begs the question of how processes are directed into specific neighborhoods. The selective fasciculation of processes onto specific axonal pathways has been demonstrated in grasshopper embryos (Raper et al. 1983). The close association of groups of processes suggests that the processes within groups may be mutually adhesive and could have selectively fasciculated during outgrowth. (The sudden loss of a closely associated neighbor may encourage a process to leave a neighborhood to seek out high-affinity processes in another neighborhood). A neuron-specific cell adhesion factor (N-CAM) has been described (Edelman 1983) that has been shown to mediatethe fasciculation of neuron processes. N-CAM is ubiquitous to neurons, but its expression has been shown to be spatially and temporarilly modulated during development, which might explain how a single adhesion factor could be used to mediate localized fasciculations of small groups of processes within a process bundle. A conceptually simpler explanation might be that there are neighborhood-specific cell adgesion factors that are expressed on closely associated cells within a neighborhood. Some of the monoclonal antibodies that have been raised to leech nervous tissue bind to fascicles of processes within larger process bundles (Hockfield and McKay 1983; K. Kotrla and C .Goodman, pers. comm.). This suggests that the processes of the fascicle share a common antigen; such an antigen could be a neighborhood-specific adhesion molecule.
We would like to conclude by proposing that the exquisitely detailed and precise patterns of synaptic connectivity that are present in the nervous system of C. elegans may arise by the coordinate operation of the three mechanisms described below.
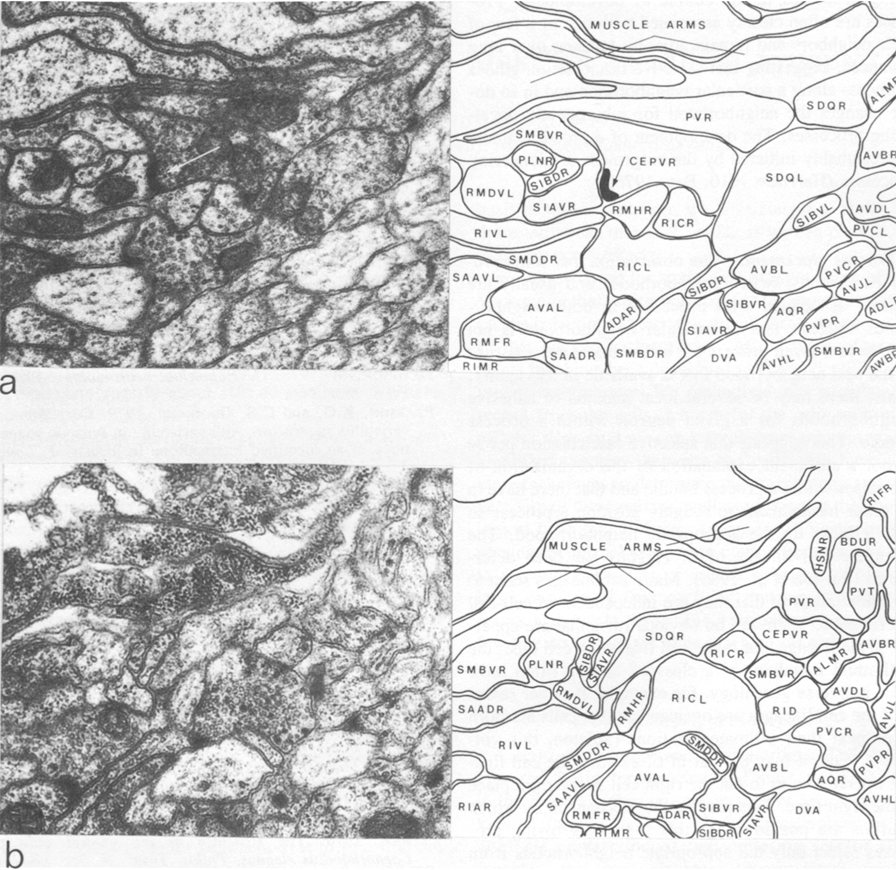
Figure 4.Comparison of two similar regions of neuropil from two different reconstructed animals. In the U-series animal, the process of CEPVR runs adjacent to that of SIAVR for some distance and makes several synapses to it (one is indicated with an arrow). In the H-series animals, these two processes are never adjacent (b) and there are no synapses between them. Magnification, 30,000x.
Intrinsic synaptic specificities. As we have discussed, synaptic specificities between classes in C. elegans are rather broad, in that a neuron of a given class could make synaptic contacts with many classes of neurons if they were all accesible. There nevertheless is a considerable class discrimination within a neighborhood. Anatomical reconstructions by themselves give no clue as to the basis of these specificities; however, it is notable that there is a strong bias against reciprocal chemical synapses between neurons and also a weaker but significant bias against simultaneous gap junctions and chemical synapses between neurons (J.G. White and E. Southgate, unpubl.). These observations suggest that there may be competing interactions between the three different types of synaptic contacts of one type or another to many of their neighbors, such interactions would reduce the amount of class discrimination that is necessary to give the patterns of synaptic contact seen within neighborhoods.
Process guidance. Little is known as yet about how processes grow into process bundles in C. elegans. It is, however, notable that nearly all process bundles have processes that run in opposite directions, yet there is no obvious spatial segregation of processes according to their direction of growth. Processes from neurons that develop postembryonically are often localized deep within process bundles and are surrounded by processes that are laid down embryonically (e.g., the ventral cord motor neurons; White et al. 1976). It is thus probable that processes can insinuate themselves between preexisting processes in the course of development. Processes are often closely associated with one or a few of their neighbors and remain adjacent to them over long distances, suggesting that selective fasiculation guides a process along a particular neighborhood and in so doing changes the neighborhood for subsequently developing processes. The development of a process bundle is presumably initiated by the outgrowth of pioneering processes (Harrison 1910; Bate 1976).
Process placement. The observation that some processes abruptly switch neighborhoods and usually are closely associated with processes in both neighborhoods suggests that a particular neighborhood is not unique to a given process but rather that a process runs in the best neighborhood that is available in its vicinity. Thus, there may be several local maxima of adhesive neighborhoods for a given neuron within a process bundle. This suggests that selective fasiculation per se is not a sufficient explanation of the organization of processes within a process bundle and that there have to be other mechanisms to roughly position a process so that it picks up the appropriate neighborhood .The complete cell lineage of C. elegans has been determined (Sulston et al. 1983). Many sublineages seem to be autonomous in that they are indepenent of cell-cell interactions. There are no obvious rules that are apparent in the lineage that relate cell lineage to cell type' the individual members of a class of neuron often have quite a diverse genealogy, for example. It rather seems as if the cell lineages are organized so that cells are born at or near their ultimate locations (Sulton, this volume). Thus, it may be that in C. elegans the cell lineage operates so as to put the right place at the right time. This may be the mechanism by which neurons are positioned so that their outgrowing processes select only the appropiate neighborhoods from all the other available neighborhoods.
Acknowledgment
We thank D.G. Albertson and J.E. Sulston for discussions and critical reading of the manuscript.
References
Albertson, D.G. and J.N. Thomson. 1976. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. 275: 299.
Bate, C.M. 1976. Pioneer neurones in an insect embryo. Nature 260: 54.
Edelman, G.M. 1983. Cell adhesion molecules. Science 219: 450.
Harrison, R.G. 1910. The outgrowth of the nerve fibre as a mode of protoplasmic movement. J. Exp. Zool. 9: 787.
Hockfield, S. and R. McKay. 1983. Monoclonal antibodies demonstrate the organization of axons in the leech. J. Neurosci. 3: 369.
Jacobson, M. 1978. Developmental neurobiology. Plenum Press, New York.
Pearson, K.G. and C.S. Goodman. 1979. Correlation of variability in structure with variability in synaptic connections of an identified interneurone in locusts. J. Comp. Neurol. 184: 141.
Raper, J.A., M. Bastiane, and C.S. Goodman. 1983. Pathfinding by neural growth cones in grasshopper embryos. J. Neurosci. 3: 31.
Sulston, J.E. and H.R. Horvitz. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 96: 110.
Sulston, J.E., E. Schierenberg, J.G. White, and J.N. Thomso. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 98: (in press).
Ward, S., N. Thomson, J.G. White, and S. Brenne., 1975. Electron microscopal reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160:313.
Ware, R.W., D. Clark, K. Crossland, and R.L. Russell. 1975. The nerve ring of the nematode Caenorhabditis elegans: Sensory input and motor output. J. comp. Neurol. 162: 71.
White, J.G., E. Southgate, J.N. Thomson, and S. Brenner. 1976. The structure of the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B 275: 327
|