
Post-Embryonic Development in
the Ventral Cord of Caenorhabditis elegans
J. E. Sultson
Philosophical Transactions of the Royal Society of London.
Series B, Biological Sciences, Vol.275, No.938. (Aug. 10, 1976), pp.287-297
Introduction -
Methods -
Results -
Discussion -
Acknowledgments -
References
56 nerve cells are added to the ventral cord and associated ganglia of Caenorhabditis elegans at about the time of the first larval moult. These cells are produced by the uniform division of 13 neuroblasts followed by a defined pattern of cell deaths. Comparison with the data in the previous paper suggests that there is a relationship between the ancestry of a cell and its function. The significance of programmed cell death is discussed.
Introduction
In the first of these three papers, White, Southgate, Thomson & Brenner (1976) have shown that the neurones in the ventral cord of Caenorhabditis elegans can be assigned morphologically to distinct classes, and that these classes recur periodically along the length of the cord. The number of cells of each class was found to be invariant within the sample studied, but there were slight variations in their order. We now ask whether any clues to the determination of the various cell classes can be found in the development of the nematode. The life cycle of C. elegans, like that of other nematodes, comprises the egg, four larval stages and the adult. The larval stages are designated L1-L4 and each ends with a moult (table 1). The sexual forms are hermaphrodite (XX) and male (XO); except where otherwise stated, the hermaphrodite form will be considered in this account.
The L1 larva which emerges from the egg is in many respects similar to the adult. The most obvious difference is in the size of the gonad, which contains only four cells. On closer examination, however, a number of other tissues are seen to contain fewer cells than in the adult; these include the gut, the hypodermis, the tail, the ventral cord and the body musculature. It is fortunate for the present study that three-quarters of the ventral cord cells appear after hatching, since the nematode is much more accessible to observation when it is no longer surrounded by the egg case.

Wessing (1953) has already demonstrated an increase in cell number in the gut, hypodermis and ventral cord during the larval development of Rhabditis anomala, a closely related nematode. The following account adds to his data by describing the detailed lineage of some of the late cells. No evidence has been found in C. elegans for the amitotic divisions which he observed in R. anomala.
Methods
(a) Culture
The culture of C. elegans has been described by Brenner (1974).
(b) Observation of living specimens
A drop of 5% agar, de-aerated by heating at 100°C, was placed on a clean microscope slide, and flattened into a layer about 0.5 mm thick by means of a second, siliconized, slide supported on spacers. Care was taken to avoid imperfections due to bubbles or dust, which might subsequently trap the nematode during a series of observations. After 2 min the siliconized slide was removed; 2 Ál of 10 % polyvinylpyrrolidone in S medium (Sulston & Brenner 1974) was placed on the agar, and a selected nematode was transferred to this drop. The centre of a 12 mm x 12 mm cover slip was very thinly coated with bacteria (E. coli, strain OP50) scraped from a plate culture, and the cover slip was lowered gently onto the agar. Excess agar was trimmed away, and the edges of the assembly were sealed with silicone grease. Most of the air bubbles were soon absorbed by the de-aerated agar, and after a short period of quiescence (due probably to anoxia) the nematode generally moved into the bacterial lawn and started to feed. Non-optimum temperature and shortage of food were found to be conducive to escape.
A Zeiss Universal microscope was equipped with a Plan x 100 objective and Nomarski differential interference contrast optics, and was kept at an air temperature of 20-22°C. Photographs were taken on Ilford Pan F 35 mm film, with a Zeiss microflash illuminator as the light source, and developed in M. & B. Promicrol.
During periods of cell division and migration, the ventral cord was observed continuously and sketched at 15 min intervals; during the much longer periods of quiescence, observations were made at 1 or 2 h intervals. When a single individual was to be followed for more than one day, it was refrigerated overnight at 6-8°C during a suitable quiescent period. This cooling was found practically to arrest development, and yet on returning to 20°C the animal continued to grow normally.
(c) Feulgen staining
Nematodes were transferred from plates by means of a paper strip or pointed stick to a small drop of 10% ovalbumin on a microscope slide; the drop was smeared out, and the slide was plunged into Carnoy's fixative. For multiple samples, the slide was first divided into zones by Indian ink lines, and was kept cool during transfers in order to avoid drying of the drops. After hydration, Feulgen staining, and dehydration, the nematodes were mounted in methyl salicylate or Depex.
Results
The development of the nematode was observed by light microscopy, using Nomarski differential interference contrast optics. This technique is non-destructive, can be applied to the animal under reasonably natural conditions, and, at least in the L1, enables every nucleus to be resolved. Various methods of restraint were tried, without success, and it was finally found best to allow the animal to move freely, though confined beneath a coverslip by the attraction of a bacterial lawn. The presence of an agar layer slows movement somewhat, but does not impede feeding.
Although photographs are useful for illustrative purposes, direct observation is the only satisfactory method for following cell division and migration. Since the cells move in three dimensions, large numbers of photographs would be needed for a complete record, and, in any case, much potential resolution is lost when photographing the low contrast Nomarski image with its shallow depth of field.
Cell boundaries cannot always be visualized by Nomarski optics, but nuclei are resolved clearly and different types can be distinguished (figures 8-14, plate I). Nerve cells have granular nucleoplasm and generally no visible nucleolus in the L1: they are indistinguishable from the supporting cell nuclei which lie anterior to the nerve ring (Ward, Thornson, White & Brenner 1975), and will be termed compact nuclei. Muscle nuclei in the L1 are ovoid, with granular nucleoplasm surrounding a spherical nucleolus; during L2 the nucleoplasm becomes smooth so that they can no longer be distinguished morphologically from ventral hypodermal nuclei. The body muscle nuclei lie in four longitudinal rows, subventrally and subdorsally. Lateral hypodermal and stem cells have large nuclei and nucleoli. Gut and gonad nuclei can be identified both by morphology and by position. The designation of the various cell types depends upon tracing them through to the adult, in which they have been identified
by reconstruction from serial section electron micrographs.
(a) The L1 larva
In the young L1, the ventral cord contains fifteen compact nuclei in a practically invariant arrangement (figure 1a). At the anterior end lies the retro-vesicular ganglion (r.v.g.), comprising twelve compact nuclei and one large nucleus with a nucleolus. The cells of the r.v.g, lie within a basement membrane (White et al. 1976); this membrane, and particularly the constraint which it imposes upon relative movement of the cells, helps to mark out the r.v.g. as an identifiable unit. At the posterior end of the ventral cord lies the pre-anal ganglion (p.a.g.); it contains six compact nuclei. Unlike the r.v.g., the p.a.g. does not have a well defined boundary, and, since it has not yet been studied by electron microscopy, there is no independent check upon the number of nerve nuclei in the vicinity. It will be seen later that the r.v.g. contains cells of the types found in the cord as well as more specialized cells; the same may also be true of the p.a.g.
In all these areas, the number of cells and their approximate arrangement are invariant. The compact nuclei found in the ventral cord, r.v.g. and p.a.g. of the young L1 will be termed juvenile (J).
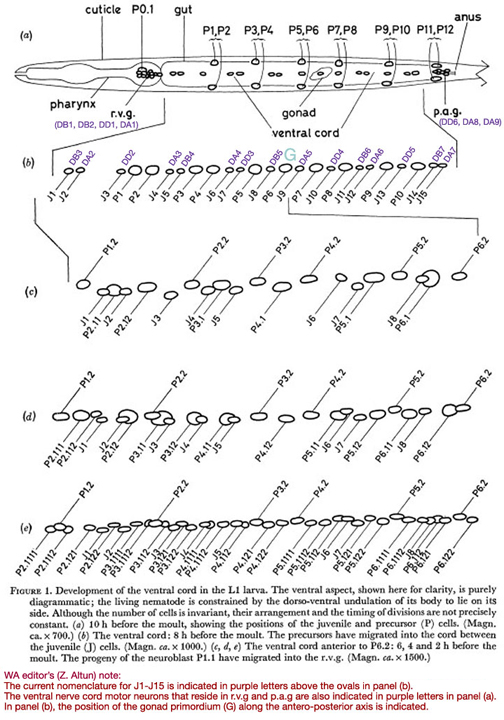 Figure 1. Development of the ventral cord in the L1 larva. The ventral aspect, shown here for clarity, is purely diagrammatic; the living nematode is constrained by the dorso-ventral undulation of its body to lie on its side. Although the number of cells is invariant, their arrangement and the timing of divisions are not precisely constant. (a) 10 h before the moult, showing the positions of the juvenile and precursor (P) cells. (Magn. ca. x 700.) (b) The ventral cord: 8 h before the moult. The precursors have migrated into the cord between the juvenile (J) cells. (Magn. ca. x 1000.) (c, d, e) The ventral cord anterior to P6.2: 6, 4 and 2 h before the moult. The progeny of the neuroblast P1.1 have migrated into the r.v.g. (Magn. ca. x 1500.)
Figure 1. Development of the ventral cord in the L1 larva. The ventral aspect, shown here for clarity, is purely diagrammatic; the living nematode is constrained by the dorso-ventral undulation of its body to lie on its side. Although the number of cells is invariant, their arrangement and the timing of divisions are not precisely constant. (a) 10 h before the moult, showing the positions of the juvenile and precursor (P) cells. (Magn. ca. x 700.) (b) The ventral cord: 8 h before the moult. The precursors have migrated into the cord between the juvenile (J) cells. (Magn. ca. x 1000.) (c, d, e) The ventral cord anterior to P6.2: 6, 4 and 2 h before the moult. The progeny of the neuroblast P1.1 have migrated into the r.v.g. (Magn. ca. x 1500.)
(b) Development in the L1
In the young L1, six symmetrically placed pairs of precursor nuclei lie ventro-laterally, between the subventral muscle nuclei and the lateral hypodermal nuclei (figure 1a). By mid-Ll there is an indication of discontinuity between the precursor cells and their hypodermal neighbours (figure 12), and at about eight hours after hatching, their nuclei begin to migrate into the ventral cord and p.a.g. The migration is preceded by the slow growth of characteristically striated areas of cytoplasm in the ventral cord; presumably, an extension of each precursor cell grows round into the cord some time before the nuclear migration. The most anterior pair migrate first, followed roughly in order by successively more posterior pairs: the same general anterior-posterior ordering applies to every phase of the development. In the case of the pairs P3/P4 and P5/P6, the left-hand precursor may finally lie either anterior or posterior to its right-hand partner, at random. Elsewhere, however, there is at least a strong tendency to a particular configuration. Thus P1 has been seen eleven times to come from the right and once possibly to come from the left. PI2 normally comes from the right. The final positions of the precursor nuclei among the juvenile nerve nuclei are not precisely constant, but a typical arrangement is shown in figure 1b.
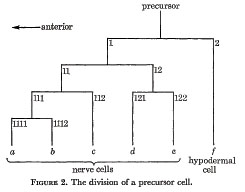
Every precursor divides as shown in figure 2. The first anterior daughter is a neuroblast, while the first posterior daughter is a ventral hypodermal cell. The axes of all the divisions are approximately parallel to the longitudinal axis of the nematode. In order to label the various cells, an anterior daughter is given its parent's number with the suffix 1, and a posterior daughter its parent's number with the suffix 2. In addition, the differentiated cells are labeled by the letters a to f as shown.
White et al. (1976) have demonstrated cytoplasmic continuity between the ventral and lateral hypodermal ridges in the adult. It is now known that the precursors are separate cells whose posterior daughters ultimately fuse with the ventral hypodermal ridge (J.G. White, personal communication).
There is one additional neuroblast, which is already present in newly hatched larvae: it is the nucleolated cell which lies at the anterior end of the r.v.g. It divides in exactly the same manner as the other neuroblasts, and will be regarded as the daughter of a hypothetical precursor P0; its actual ancestry, however, is as yet unknown.
During the period of division, the neuroblasts and their progeny migrate freely past the juvenile cells and the hypodermal cells. Except in the r.v.g., however, the progeny of one neuroblast do not migrate past those of another; nor, except at the anterior end of the r.v.g. and the posterior end of the p.a.g., do the progeny of a single neuroblast migrate past one another.
The direction of movement of each nucleus at a given time is quite reproducible; the extent of movement, however, is variable and seems to depend upon local pressures. It is also noteworthy that the entire process has a fixed hand. For example: J3 always moves far to the right; J1 and J2 move slightly right and ventrally; all hypodermal cells move gradually to the left. This asymmetry may be determined by the previous existence of a hypodermal ridge to the left of the L1 ventral cord, and the consequent restriction of the muscle synapses to the right of the cord. Typical stages in the development of the anterior part of the ventral cord are shown in figure 1 and figures 8-11, plate 1.
Towards the end of the period of division, certain cells die. Their death is signalled by a loss of contrast in the granularity of the nucleoplasm, followed by a sudden increase in the refractility of the nucleus (figure 13, plate 1). This stage persists for a few minutes, and then the refractility fades with erosion of the nuclear outline. In crowded regions other nuclei completely obliterate the site, but in some places a permanent gap remains; in either case, no trace of the dead cell's nucleus can be detected by Feulgen staining. The pattern of cell death in the hermaphrodite differs from that in the male (table 2), but no individual variation has been seen.

Cell deaths can be visualized in another way, by using the mutant E1392 (nuc-1, X) which lacks the principal endodeoxyribonuclease. In this mutant, high molecular weight DNA is degraded much more slowly than usual, and, after Feulgen staining, the approximate site of each cell death is marked by a 0.5 Ám dot (figure 15).
With one exception, the ventral hypodermal cells do not usually divide in the L1. The exception is P12f whose division is followed after a short time by the death of its posterior daughter.
A typical timetable for the division of three of the precursors is shown in figure 3; the division times in the rest of the cord lie between these extremes. As indicated in figure 3, the steady pumping of the pharynx ceases rather abruptly some 2 h before the L1 moult. About 15 min later a variable number of gut cells divide; the most anterior six never normally divide, and any of the most posterior four may also fail to divide.
(c) Later development
In the hermaphrodite, no further division or rearrangement of the ventral nerve nuclei has ever been noticed. In particular, two individuals have been followed through to the adult stage, with observations at sufficiently frequent intervals that any changes should have been detected; the only development is a Iongitudinal stretching, so that some nuclei which are initially beside one another become separated. At first sight P6c and P7c appear exceptional in that they move past their neighbours towards the developing vulva. The reason for this behaviour is that they alone enter the left-hand branch of the ventral cord as it forks around the vulva. Their consequent lateral displacement means that they no longer have a defined position relative to the other nuclei. The remaining nuclei of the ventral cord eventually become linearly arranged, but in the r.v.g. and the p.a.g. some lateral stacking persists into the adult. The hypodermal nuclei, on the other hand, move to the left of the line of nerve nuclei, and in some places migrate freely past them. In the L3, three hypodermal nuclei (P5f P6f and P7f) divide as shown in figure 4, and their progeny form the vulva. The number of free hypodermal nuclei is maintained by division of P3f, P4f and P8f.
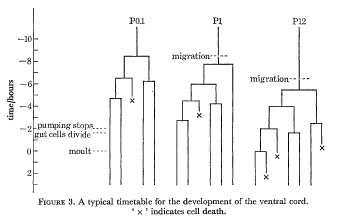 Figure 3. A typical timetable for the development of the ventral cord. ' x ' indicates cell death.
Figure 3. A typical timetable for the development of the ventral cord. ' x ' indicates cell death.
Events in the male are quite different. Shortly before the L3 moult the c nuclei (with the exception of P2c and P12c) divide once; the divisions take place to the left of the ventral cord, but afterwards the daughters insert themselves between the other nerve nuclei giving a linear array once more. The hypodermal cells in the middle of the cord do not divide, but P10f and P11f divide in the L3 and participate in forming the specialized structures of the male tail.
(d) The adult
Figure 5 indicates the extent of variation in the arrangement of nerve nuclei in the adult hermaphrodite ventral cord. It will be seen that the differences are restricted to the positioning of the juvenile cells in the fixed pattern of late cells. Examples of cell arrangements in the r.v.g. and p.a.g. are shown in figures 6 and 7.
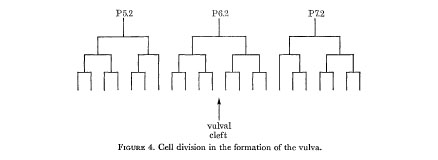 Figure 4. Cell division in the formation of the vulva.
Figure 4. Cell division in the formation of the vulva.
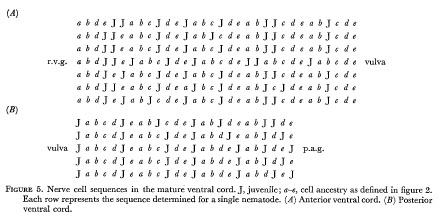 Figure 5. Nerve cell sequences in the mature ventral cord. J, juvenile; a-e, cell ancestry as defined in figure 2. Each row represents the sequence determined for a single nematode. ( A )Anterior ventral cord. (B) Posterior ventral cord.
Figure 5. Nerve cell sequences in the mature ventral cord. J, juvenile; a-e, cell ancestry as defined in figure 2. Each row represents the sequence determined for a single nematode. ( A )Anterior ventral cord. (B) Posterior ventral cord.
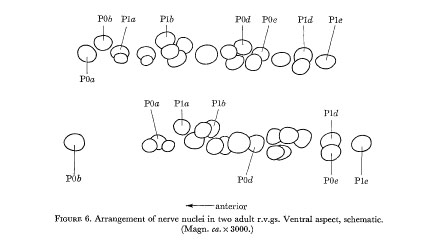 Figure 6. Arrangement of the nerve nuclei in two adult r.v.gs. Ventral aspect, schematic. (Magn. ca. x 3000.)
Figure 6. Arrangement of the nerve nuclei in two adult r.v.gs. Ventral aspect, schematic. (Magn. ca. x 3000.)
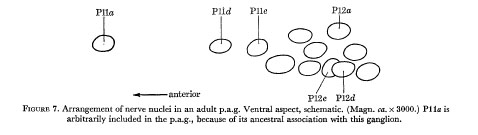 Figure 7. Arrangement of nerve nuclei in an adult p.a.g. Ventral aspect, schematic. (Magn. ca. x 3000.) Plla is arbitrarily included in the p.a.g., because of its ancestral association with this ganglion.
Figure 7. Arrangement of nerve nuclei in an adult p.a.g. Ventral aspect, schematic. (Magn. ca. x 3000.) Plla is arbitrarily included in the p.a.g., because of its ancestral association with this ganglion.
Discussion
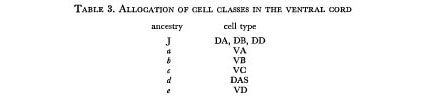
We first note that the variation in the final position of the juvenile cells in the ventral cord provides a natural explanation for the observed variation in the order of cell classes (White et al. 1975). Furthermore, the periodicity in ancestry of the late cells corresponds very well to the periodicity in five of the cell classes. If we suppose that there is a one-to-one correspondence between the ancestry of a late cell and its class, we find that only one self-consistent set of allocations can be made within the observed limits of variation (table 3).


The reason for comparing the lineage data with previously established electron microscopic assignments is that sectioning the ventral cord and tracing neuronal connections is extremely time-consuming. It is therefore impracticable to test the proposed allocations exhaustively. However, the expected relationships were confirmed directly for a short stretch of ventral cord in one animal (table 4).
The non-linear arrangement of cells in the r.v.g. precludes comparison with known assignments. The lineage of the late cells in two r.v.gs was therefore determined and the cells were identified by serial section electron microscopy. The allocations are identical for the two r.v.gs, but differ in the case of three cells from those for the ventral cord (table 5).
The failure of P1a to become a VA cell draws a distinction between precursors P1 and P2, which were symmetrically arranged in the young L1. In the male there is an additional difference between them in that P1c dies but P2c survives. This may mean that P1 is already determined before migrating into the ventral cord and that it is thereby constrained to enter the cord anterior to P2. Alternatively, the fates of cells may be partly determined by local influences.
An interesting feature of the allocations given in table 3 is that in the adult the axons of the juvenile cells innervate solely the dorsal cord. It seems unlikely that the L1 entirely lacks ventral innervation, and indeed White has shown (unpublished observations) that in the young L1 the DD cells innervate the ventral cord instead of the dorsal cord. The events at the L1 moult, therefore, include the loss of old neuronal connections as well as the addition of new ones.
It appears then, that the pattern of division shown in figure 2 partially determines the function of the cells to which it gives rise. There are two extreme ways in which this could occur. First, a distinction may be made between the anterior and posterior daughter at each division, and this information may be accumulated to determine the properties of the differentiated cells. Secondly, the position of a cell in the final array may be the determining factor. It is hoped eventually to distinguish between these possibilities by selective destruction of cells during the division process. For the present, the following observations tend to support the first mechanism.
(1) The division pattern itself requires anterior and posterior daughters to be distinguished in some way.
(2) The variable movement of the dividing nuclei among the juvenile nuclei suggests that specific cytoplasmic bridges between the late cells would be required for the second mechanism. However, the death of a c cell frequently precedes the generation of the corresponding a and b cells, indicating that at least the a/b determination is independent of contact with the other cells.
If, indeed, there is lineal determination of cell function, the phenomenon of programmed cell death takes on particular significance. For, if each precursor is committed to producing a fixed array of five cell types, the destruction of a cell whose function is not required at a given location is entirely reasonable. Mechanisms of this sort provide a possible explanation for the widespread occurrence of 'histogenetic' cell death in embryogenesis (Saunders 1966).
The pattern of c cell death is just one aspect of the different roles played by these neurones in the two sexes. White et al. (1976) have shown that, in the hermaphrodite, VC cells make relatively few synapses with the body muscles but form an important input to the muscles of the vulva; in the male, on the other hand, the far more numerous VC cells have no vulva to innervate. A possible interpretation of these observations is that in the male the VC neurons are excitatory to the body muscles, being responsible for the characteristic arching reflex; in the hermaphrodite this function is not required, but some of the VCs are retained and employed in control of the vulva. Such sexual re-allocation parallels the positional re-allocation seen in the r.v.g.
In a general way, it is also possible to explain the loss of a VA cell anteriorly and a VB cell posteriorly. Since the axons of VA cells project forwards to the next most anterior set of muscle synapses, and those of VB cells project backwards (White et al. 1976), end effects of some kind are inevitable. We may therefore regard the re-allocation of an a cell in the r.v.g. and the death of a b cell in the p.a.g. as equivalent events. It is not clear, though, why two cells should be affected at each end, especially in view of the re-allocation of Pod as a VA in the r.v.g. It will be interesting to see whether P12d or P12e is re-allocated as a VB in the p.a.g.
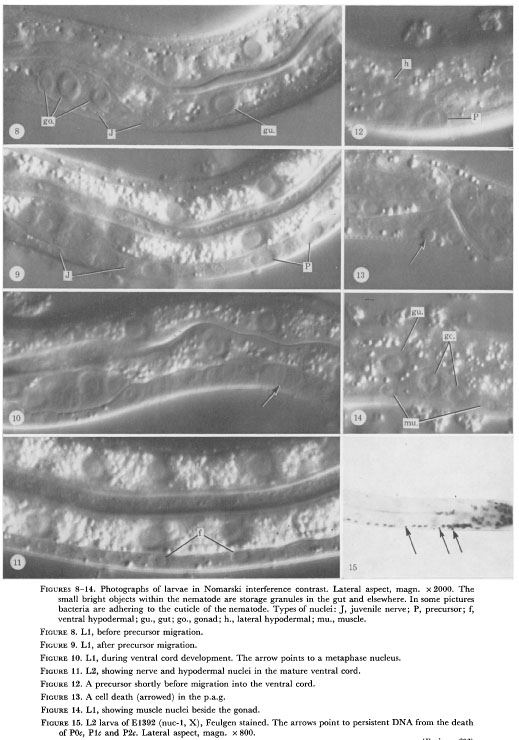 Figure 8-14. Photographs of larvae in Nomarski interference contrast. Lateral aspect, magn. x 2000. The
small bright objects within the nematode are storage granules in the gut and elsewhere. In some pictures
bacteria are adhering to the cuticle of the nematode. Types of nuclei: J, juvenile nerve; P, precursor; f,
ventral hypodermal; gu., gut; go., gonad; h., lateral hypodermal; mu., muscle.
Figure 8-14. Photographs of larvae in Nomarski interference contrast. Lateral aspect, magn. x 2000. The
small bright objects within the nematode are storage granules in the gut and elsewhere. In some pictures
bacteria are adhering to the cuticle of the nematode. Types of nuclei: J, juvenile nerve; P, precursor; f,
ventral hypodermal; gu., gut; go., gonad; h., lateral hypodermal; mu., muscle.
Figure 8. L1, before precursor migration.
Figure 9. L1, after precursor migration.
Figure 10. L1, during ventral cord development. The arrow points to a metaphase nucleus.
Figure 11. L2, showing nerve and hypodermal nuclei in the mature ventral cord.
Figure 12. A precursor shortly before migration into the ventral cord.
Figure 13. A cell death (arrowed) in the p.a.g.
Figure 14. L1, showing muscle nuclei beside the gonad.
Figure 15. L2 larva of El392 (nuc-1, X), Feulgen stained. The arrows point to persistent DNA from the death
of P0c, P1c and P2c. Lateral aspect, magn. x 800.
Acknowledgments
I am most grateful to Roger Freedman and Simon Pickvance for introducing me to Nomarski microscopy, to Sydney Brenner, John White and Bob Horvitz for discussion and to Marilyn Dew for assistance. Eileen Southgate, Nicol Thomson and John White identified the cells in animals of known lineage.
References
Brenner, S. 1974 The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
Saunders, J. W. 1966 Death in embryonic systems. Science, N.Y. 154, 604-612.
Sulston, J. E. & Brenner, S. 1974 The DNA of Caenorhabditis elegans. Genetics 77, 95-104.
Ward, S., Thomson, N., White, J. G. & Brenner, S. 1975 Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. comp. Neurol. 160, 313-337.
Wessing, A. 1953 Histologische Studien zu den Problemen der Zellkonstanz: Untersuchungen an Rhabditis anomala P. Hertwig. 2001. Jahrb. 73, 69-102.
White, J. G., Southgate, E., Thomson, N. & Brenner, S. 1976 Phil. Trans. R. Soc. Lond. B 275, 327-348.
|