
Neuronal Cell Lineages in the Nematode Caenorhabditis elegans
J. E. Sulston
Laboratory of Molecular Biology, Medical Research Council Centre, Hills road, Cambridge, CB2 2QH, Engand
Introduction - Sources of the Nervous System - Lineage Patterns -
Programmed Cell Deaths - Migration - The Formation of Sensilla - Cell Lineage and Neurotransmitters - Cell-cell Interaction - Generation of Analogs and Unique Cells - Comparisons Between Species - Cells Having Multiple Functions - Conclusion - Acknowledgments - References
One of the attractive properties of the small nematode Caenorhabditis elegans (Fig.1) is that the divisions, migrations, and deaths of all its cells can be followed continuously in living individuals from conception to maturity. By means of Nomarski differential interference contrast microscopy, the entire cell lineage of the animal has been traced (Sulston and Horovitz 1977; Kimble and Hirsh 1979; Sulston et al. 1983) and has been related to the anatomy as reconstructed by electron microscopy (Ward et al. 1975; Ware et al. 1975; Albertson and Thomson 1976; White et al. 1976; Sulston et al. 1980; J.G White et al. 1976., in prep. and this volume). The early embryonic lineages were accurately described at the turn of the century (for review, see Chitwood and Chitwood 1974), but, dependent as the observations then were upon comparisons between embryos fixed and stained at different ages, they could not be extended to later divisions and movements, or to postembryonic development. In the assignments of early embryonic cells to tissues, the findings of these pioneers have broadly been confirmed; in the later lineages, however, an unpredicted complexity of detail has emerged.
In this review, my aim will be to summarize the principal findings concerning the ontogeny of the nervous system. Special attention will be paid to the embryonic stage, postembryonic neurogenesis having been recently reviewed by Horvitz (1981).
Before proceeding to an examination of the actual data, it is well to ask: Does the lineage have any significance beyond mere cell proliferation? To go some way towards answering this question, a number of cell ablation experiments, in which chosen cells are killed by means of a laser microbeam (Sulston and White 1980; Kimble 1981), have been carried out. Only a small proportion of the technically feasible experiments have been performed, but on the basis of this limited sample it appears that the nematode is highly mosaic in character: With certain well-defined exceptions, killed cells are not replaced from any other source and the differentiation of their neighbors is unaffected. Thus, if a given precursor is killed, subsequent examination of the animal reveals in most cases that all the cells usually derived from that precursor are missing and that all other cells are present. Particular examples of such experiments are described below; for the moment, it is sufficient to note that the general bona fides of the lineage, as an instrument of specific cell differentiation, has been established.
Sources of the Nervous System
Figure 2 shows the earliest divisions of the zygote, by which the five somatic "founder" cells are generated. The founder cells were recognized classically as having unique characters, in terms of divison rate, behavior of their descendants at gastrulation, and (so far as could be ascertained at that time) the tissues to which they contribute (Chitwood and Chitwood 1974). They are separated by a series of unequal divisions from the stem-cell-like germ line. The initial divisions of the founder cells themselves, on the other hand, are equal (The term "equal" means that the daughters are equal in size; "equational" means that they have the same putative fate), and, with the exception of AB, are approximately equational in terms of subsequent development. More recent work has confirmed these observations and has demonstrated clearly that the unique character of the cell line derived from each founder is autonomously maintained (Laufer et al. 1980).
At gastrulation, the descendants of E are the first to enter the interior, followed by those of MS, P4, D, some of C, and finally some of AB. The remainder of AB and C stay on the outside until the nervous system is formed.
Embryonically, neurons are derived largely from AB (200 in the hermaphrodite), but a few come from MS (6) and C (2). After hatching, additional neurons are generated by a number of blast cells all of which are descendants of AB (in Caenorhabditis; the realted nematode Panagrellus has one postembryonic neuroblast that is descended from C-see below).
It is apparent from Figure 2 that at an early stage there is a broad, but not an absolute, seperation of mesodermal from ectodermal tissues. Thus, some neurons arise from a founder cell that behaves mesodermally at gastrulation and generates predominantly muscles. Conversely, some muscles arise from the mainly ectodermal founder cell AB. The latter observation finds a parallel in vertabrate embryology, in the nematode, seperation of muscles from neurons can take place as late as the terminal division (see Fig.7)
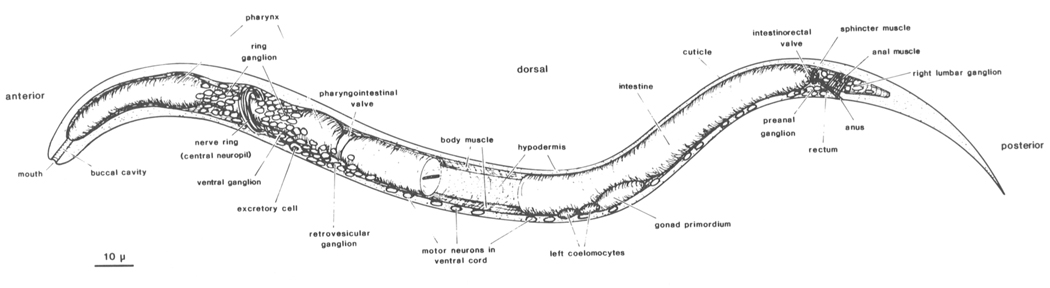 Figure 1. Newly hatched larva of C. elegans: body wall cut along dorsal and ventral midline, portion of intestine removed to show inner surface of body wall with longitudinal bands of body muscle. Principal parts of nervous system represented by neuronal nuclei. C. elegans has two sexual forms (self-fertilizing hermaphrodite and male) and grows to an adult length of 1-1.5 mm. During postembryonic development, a midventral vulva is formed in the hermaphrodite, whereas the male tail becomes a specialized copulatory bursa surrounded by sensory rays. (Reprinted, with permission, from Sulston et al. 1983.) Figure 1. Newly hatched larva of C. elegans: body wall cut along dorsal and ventral midline, portion of intestine removed to show inner surface of body wall with longitudinal bands of body muscle. Principal parts of nervous system represented by neuronal nuclei. C. elegans has two sexual forms (self-fertilizing hermaphrodite and male) and grows to an adult length of 1-1.5 mm. During postembryonic development, a midventral vulva is formed in the hermaphrodite, whereas the male tail becomes a specialized copulatory bursa surrounded by sensory rays. (Reprinted, with permission, from Sulston et al. 1983.)
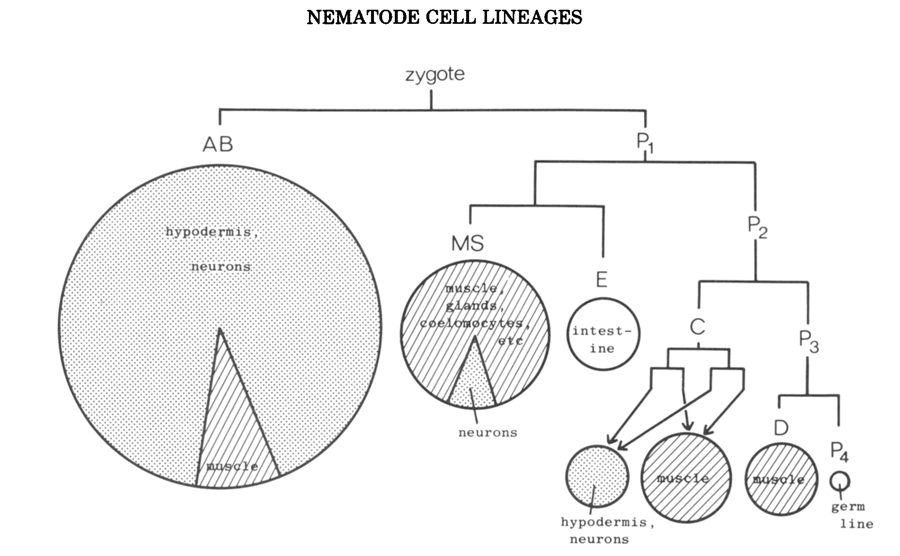
Figure 2. Early division pattern of the embryo of C. elegans, showing orgin of the founder cells. Areas of circles and sectors of circles are proportional to numbers of cells in the various categories; typically ectodermal tissues are stippled, mesodermal tissues are shaded, others are left blank. (Reprinted, with permission, from Sulston et al. 1983.)
Lineage Patterns
Turning to the terminal portions of the lineage tree, one is at first struck by their complexity (e.g., Fig.4). In searching for patterns in them, we can discern two modes of organization: (1) clonal and (2) repetitious.
Clonal development.
Clones of cells of a single type are found in the intestine, the germ line, and the body muscle. In neurogenesis, however, such clonal organization is not found. The nearest approximation is the semiclonal generation of two classes of neuron in the juvenile ventral cord (Fig.3). It is tempting to compare this situation with that of the seperation of ectodermal from mesodermal tissue; perhaps in both cases, a more primitive ancestor contained homogeneous clones, of which particular members were subsequently respecified for new functions. The absence of homogeneous clones in the nervous system may well reflect its need for a great diversity of cell types.
Repetitious development. More common in neurogenesis is the repeated sublineage-i.e., a pattern of cell division that is found at several points in the lineage and gives rise to more or less the same array of cell types at each appearance. Some examples are shown in Figure 3. Repetition of this sort is much more pronounced in postembryonic than in embryonic development. This distinction may perhaps reflect in part the greater evolutionary antiquity of embryogenesis, repetitious elements that would once have been apparent having been largely obscured by later modifications (discussed by Chalfie et al. 1981). Even in the examples cited, local variations in the repeated sublineages are apparent (see legend Fig.3), but they are too slight to obscure the underlying homologies. Bate et al. (1981) have similarly observed local variation in a segmentally repeated sublineage of the grasshopper.
A special case of repetitious development is the parental reiteration, or stem cell lineage, in which a cell divides to yeild one daughter like itself and one that is different; the parentlike daughter then divides in the same manner, and so on. Reiterative lineages are not seen overtly in neurogenesis, but evidence from the mutant unc-86 suggests that they underlie at least some neural sublineages (Chalfie et al. 1981; Horvitz, this volume).
Programmed Cell Death
During the development of C. elegans, one in eight of all cells produced subsequently dies. The subject has recently been reviewed by Horvitz et al. (1982a).
These programmed cell deaths are found predominately in the nervous system, are predictable, and commonly occur 0.5-1 hour afterthe doomed cells are born. They seldom depend upon interaction with neighboring cells, although the latter are responsible for the phagocytosis of the corpses (Hedgecock et al. 1983). In particular, the deaths are not a consequence of the production of an excess of cells of one class, followed by the destruction of those that fail to find synaptic partners (for review, see Jacobson 1978). Rather, they seem to be an integral part of the determination by cell lineage, either representing cell types that were useful at one time but are now unwanted or being the means for the local modification of sublineage (see above).
A particularly clear example of the latter role for cell death is seen in the sexual dimorphism of the embryo. All embryos generate the same set of six sex-specific neurons, which begin to differentiate in characteristic ways (migration, formation of processes, specific attachments to other cells). Then, at a particular point in the development of the hermaphrodite embryo, the four male-specific neurons abruptly die; conversely, in the male embryo the two hermaphrodite-specific neurons die.
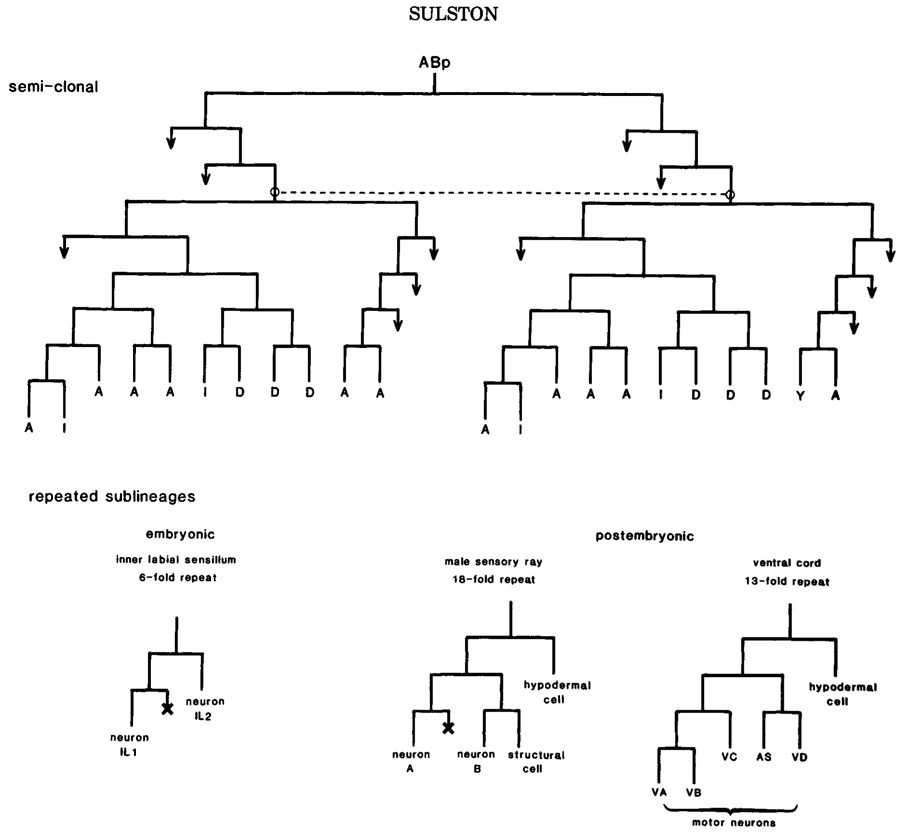
Figure 3. Semiclonal and repetitious development in the nervous system. (Above) Origin of some juvenile motor neurons; (I) interneurons; (Y) motor neuron in hermaphrodite, blast cell in male. Dotted line links homologous analogs. (Below) Neuronal sublineages. Inner labial sublineage is invariant; see Fig.4 for contexts. Other sublineagesdisplay local and sexual variation, as follows. Variations in sensory rays: neuron A dopaminergic (6X), no A/B distinction (2X). Variations in ventral cord: first division absent (1X), VA modified (2X), VB dead (2X), VC dead (7X hermaphrodite, 2X male), VC division (11X male), AS modified (1X), hypodermal cell division (1X). In this and other lineage charts, a cross designates cell death, and an arrow designates further division.
Migration
In general, each neuron is born close to the final position of its cell body. A few, however, undergo relatively long-range migrations; for example, some juvenile neurons travel half the length of the embryo (30 um) and in the young larva one pair of neuroblasts migrates 50 um (Sulston and Horvtiz 1977). These migrations involve the free crawling of rounded cells.
Another sort of movement is seen in the formation of many of the sensilla, particularly the anterior sensilla in the developing embryo and the tail sensilla in the maturing male. The sensory neurons, together with their supporting cells, come to lie just beneath the cuticle, and form attachments to one another and to the surrounding hypodermis; the cell bodies then retreat into the central nervous system, laying down their sensory and glial procees as they go. Clearly, this represents one solution to the pathfinding problem for neuronal processes. However, it is not the only one adopted by the nematode, because the growth of the efferent processes of the same neurons cannot take place in the same way. Furthermore, the cell bodies of one class of sensory neurons (the amphids) do not approach the surface but project their processes to the surface from some distance away.
The Formation of Sensilla
The nervous system is, of course, a single interconnected whole, but certain groupings of cells in it are particularly closely knit together. Among these are the sensilla.
A canonical sensillum (Ward et al. 1975) comprises one or more neurons, and two supporting cells named the sheath (surrounding the neurons) and the socket (surrounding the sheath and attached to the hypodermis); adjacent components are sealed together by desmosomes. The anterior sensilla, formed embryonically, all follow this pattern; additional anterior sensory neurons, found in older animals to lie more or less apart from any supporting cells, start their existence as accesories to the true sensilla. Other sensilla are more variable and may have either fewer or more supporting cells.
Some senilla, especially those formed late, are generated by a single mother cell. Good examples are the postdeirid (Fig.4), the male hook (Fig.5), and the male sensory rays (Fig.3), the latter showing the further regularity of an 18-fold repeated sublineage. This style of development is similar to that described for insect bristles by Lawrence (1966). In marked contrast are the sensilla formed early, which, despite the consistency of their structure, are assembled in a piecemeal (though reproducible) manner from distantly related cells (Fig.4) with only limited elements of repetition.
A trivial reason for the greater prevalence of dedicated mother cells in the formation of the late sensilla might be that the cells are more spread out at that time and fewer of them are dividing. However, this view is contradicted by the example of the male rays (late, crowded, but formed by mother cells). It seems more likely that the late sensilla have been added fairly recently in the course of evolution, and that their lineages have not yet undergone secondary modifications and rearrangements.
How are the sensory cells specified? Are their properties a function of ancestry, or are they, as appears to be the case for insect ommatidia (Lawrence and Green 1979), determined only after assembly? A variety of laser ablation studies, in which internal markers of the cells are scored (e.g., dopamine [Sulston et al. 1975], ciliary morphology, striated rootlets [Sulston et al. 1980]), have indicated that cell type is specified independently of assembly. However, assembly itself, at least in terms of neuron-socket relationships, seems to be less tightly controlled. Thus, by removal of an appropraite precursor, a neuron-sheath assembly can be made to occupy the wrong socket (Sulston et al. 1983). Such a switch can also occur spontaneously in males, whose posterior sensory rays not infrequently share a common structural cell (the term used for the single supporting cell of this sensillum) (Sulston et al. 1980).
Local influences are known to affect the branching pattern of one of the mechanosensory neurons, called PVM. Taking advantage of a mutant in which PVM is variably positioned, Chalfie et al. (1983) have shown that only when its axon grows forward to the region of the nerve ring is a side branch, with accompanying synapses, formed.
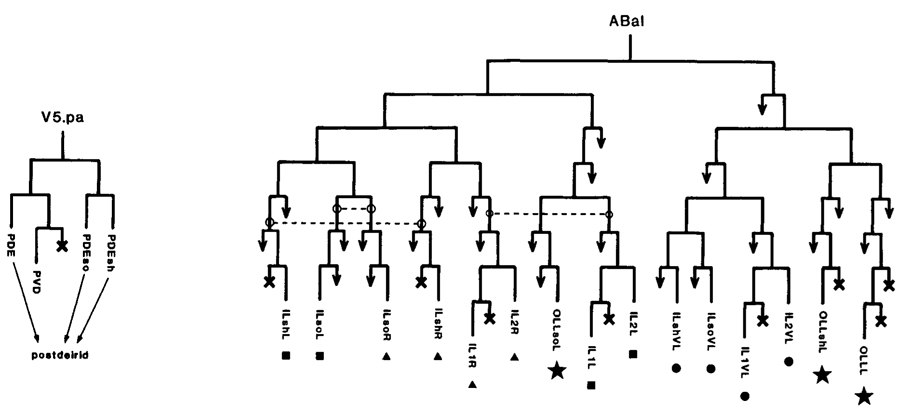
Figure 4. Lineages of some sensilla. (Left) Postdeirid; one generated on each side of the animal; mother cell yeilds sensillum and one nonassociated neuron; PDE is dopaminergic sensory neuron. (Right) A fragment of the embryonic lineage; cells giving rise to each of four sensilla are marked with the same symbol; dotted lines link analogous precursors. In cell names: (so) socket; (sh) sheath; (last letter "L" or "R") a member of a symmetrical pair, left or right, respectively.
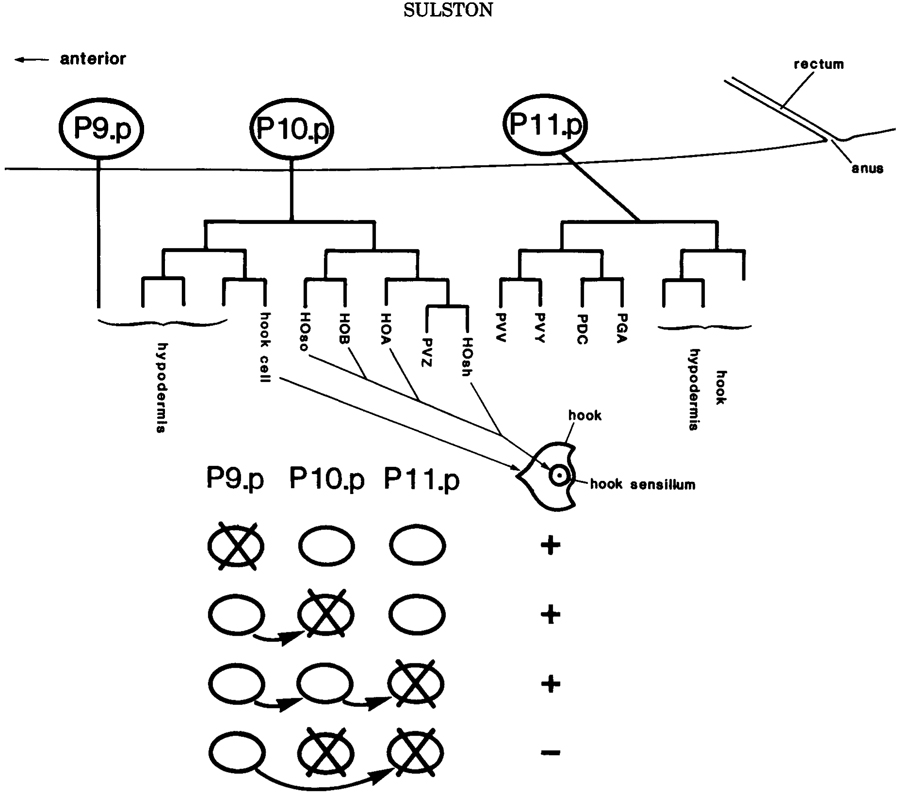
Figure 5. Preanal equivalence group in the male. Schematic lateral view showing the three memebers of the group (P9.p, P10.p, P11.p) and the lineages to which they give rise in the intact animal. The hook and its sensillum, characteristic of the P10.p lineage, are depicted in ventral view. The table beneath summarizes the replacement regulation observed after four different cell ablation experiments: for example, after the ablation of P10.p, P9.p moves into place and generatesa typical P10.p lineage.
Cell Lineage and Neurotransmitters
A number of putative neurotransmitters have been demonstrated or postulated to occur in C. elegans. Of these, two have been localized histochemically, viz. dopamine (8 cells in the hermaphrodite, 14 in the male; also identified chemically; Sulston et al. 1975) and serotonin (2 cells concerned have any special lineal relationships.
The answer appears to be that they do not, for they are scattered throughout the lineage and their nearest neighbors do not contain the same transmitter. For example, the ray sublineage is illustrated in Figure 3; six of the type-A neurons contain dopamine but are otherwise indistinguishable from the majority of their homologs in the other rays; none of the type-B neurons are dopaminergic. Again, the sensory neuron PDE contains dopamine, whereas its neice PVD does not (Fig.4). In this case, a little known of the control mechanism: Evidence from the mutants unc-86 (Chalfie et al.1981) and particularly ced-3 (Horvitz et al.1982a) indicates that dopamine synthesis is turned on in both PDE and its sister, but is then turned off specifically in PVD. Finally, the origin of the putative serotonergic neurons, NSML and NSMR, is shown in Figure 6b.
Cell-cell Interaction
Thus far, the mosaic, or cell autonomous, aspect of the C. elegans lineage has been emphasized, because that is the principal mode. There are, however, a number of known episodes of direct cell-cell interaction, most notably in young larvae, and probably more will be discovered. These involve groups of two to six cells that initally have the same developmental potential (and are therefore termed "equivalent") but subsequently undergo different fates (Sulston and White 1980; Kimble 1981). The existence and timing of the interactions responsible for their determination can be demonstrated by cell-killing experiments.
In several cases, such "equivalence groups" give rise to neuroblasts, and an example is shown in Figure 5. The sublineages generated by theneuroblasts themselves are generally cell autonomous and appear no to depend upon further cell interaction, except at the level of assembly (see above).
As well as the placement regulation characteristic of equivalence groups (Fig.5), there are various cases of induction of on type of cell by another. The best characterized of these involve the postembryonic gonad and ventral hypodermis (Kimble 1981; Kimble and White 1981), but there are also indications of long range influences in late embryogenesis that affect morphogenesis (J. Priess, pers. comm.).
In all of these interactions, the choices open to a given cell seem to be quite limited - probably no more than three at one time.
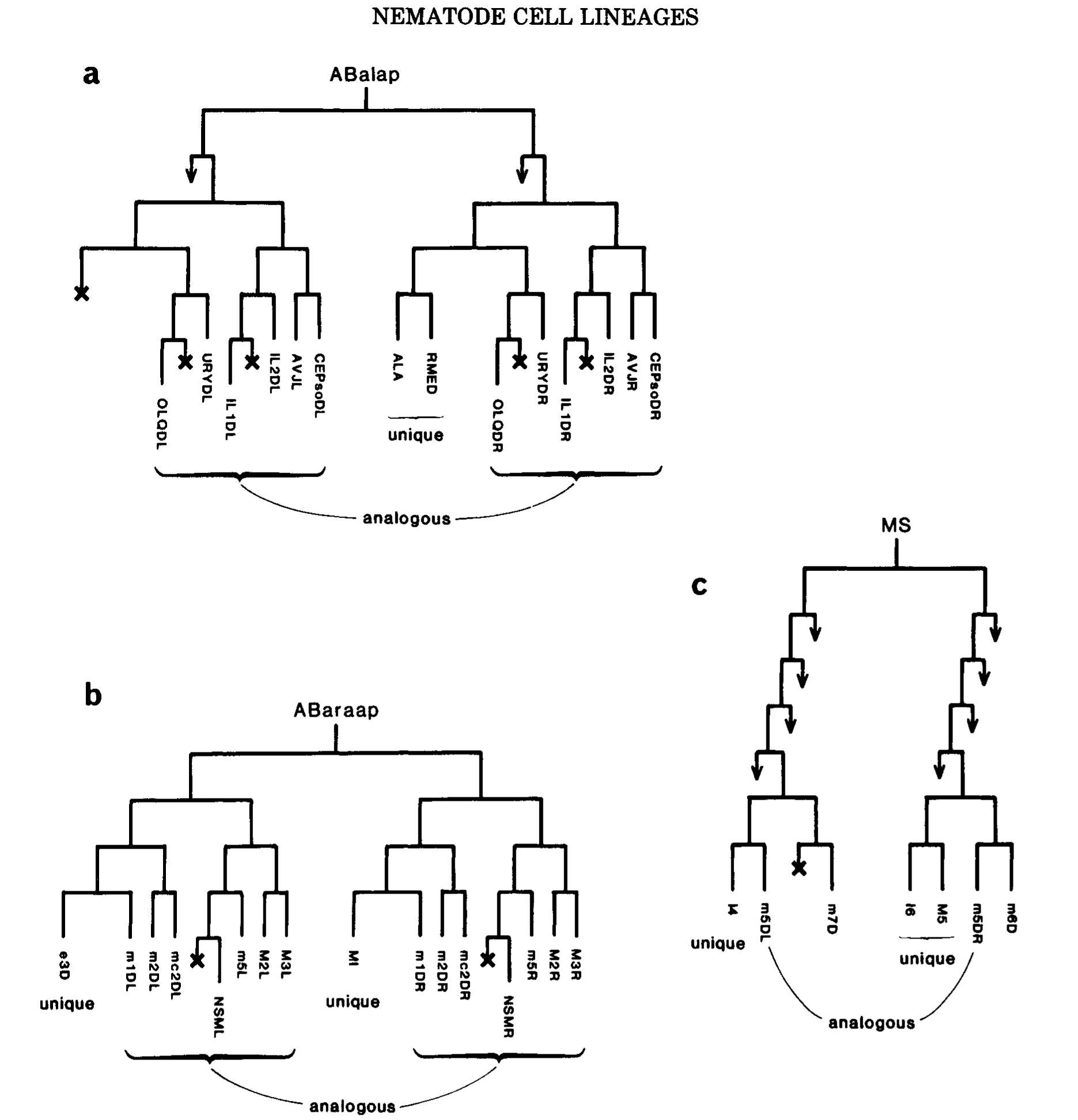
Figure 6. Generation of unique cells. (a) Unique blast cell, homolog dies. (b) Two diiferent unique cells from a pair of homologs. (c) Phase shift: homolog of muscle m5DL is the unique neuron M5, homolog of muscle m5DR is a cell death. The cells shown in b and c form part of the pharynx, and are named as follows (Albertson and Thomson 1976): (m, followed by a number) a muscle; (mc, followed by a number) a marginal (i.e., structural) cell; (first letter upper case) a neuron. Note close relationships between neurons and muscles, including two pairs of sisters (MI/m1DR; I4/m5DL).
Generation of Analogs and Unique Cells
At various times and places during development, it is possible to identify analogous precursors-i.e., cells that have the same potential destiny. Analogs include: (1) equivalent cells, defined above; (2) precursors of repeated sublineages; (3) most generally, all pairs of precursors with identical roles on the left and right sides of the animal.
How are such analogs generated? In many cases (particularly those of the third category), they are found in identical positions in the division patterns of a pair of sister cells-that is to say, they are homologous and their analogy can be readily understood in terms of an identical inheritance (Figs.3, 4, and 7). In other cases (most of those in the first two categories, and also some symmetrical pairs in the embryo), nonhomologous analogs are found-that is to say, cells of different ancestry acquire identical edvelopmental potentials. Examples can be seen in Figures 4 and 7. In most of the cases studied by cell ablation, the determination of nonhomologous analogs seems to be independent of their immediate neighbors for some time before the analogy becomes apparent. If this interpretation is correct, it implies that quite elaborate information is segregated accurately, without visible manifestation, in the course of the lineage.
The converse of the generation of analogs is the generation of unique cells. One context in which this occurs is the equivalence group, in which cells can be selected for unqiue fates by interaction with on another. Another context is found in the embryo, where numerous unique cells are generated from predominantly symmetrical lineages; thus, pairs of nonanalogous homologs can be recognized. For a single homologous pair, the end product may be either two different unique cells or one unique cell and one death (Fig.6a,b). In places, however, the generation of unique cells is more complex and involves "phase shifts" in the ancestry of analogous cell pairs as well (fig6c).
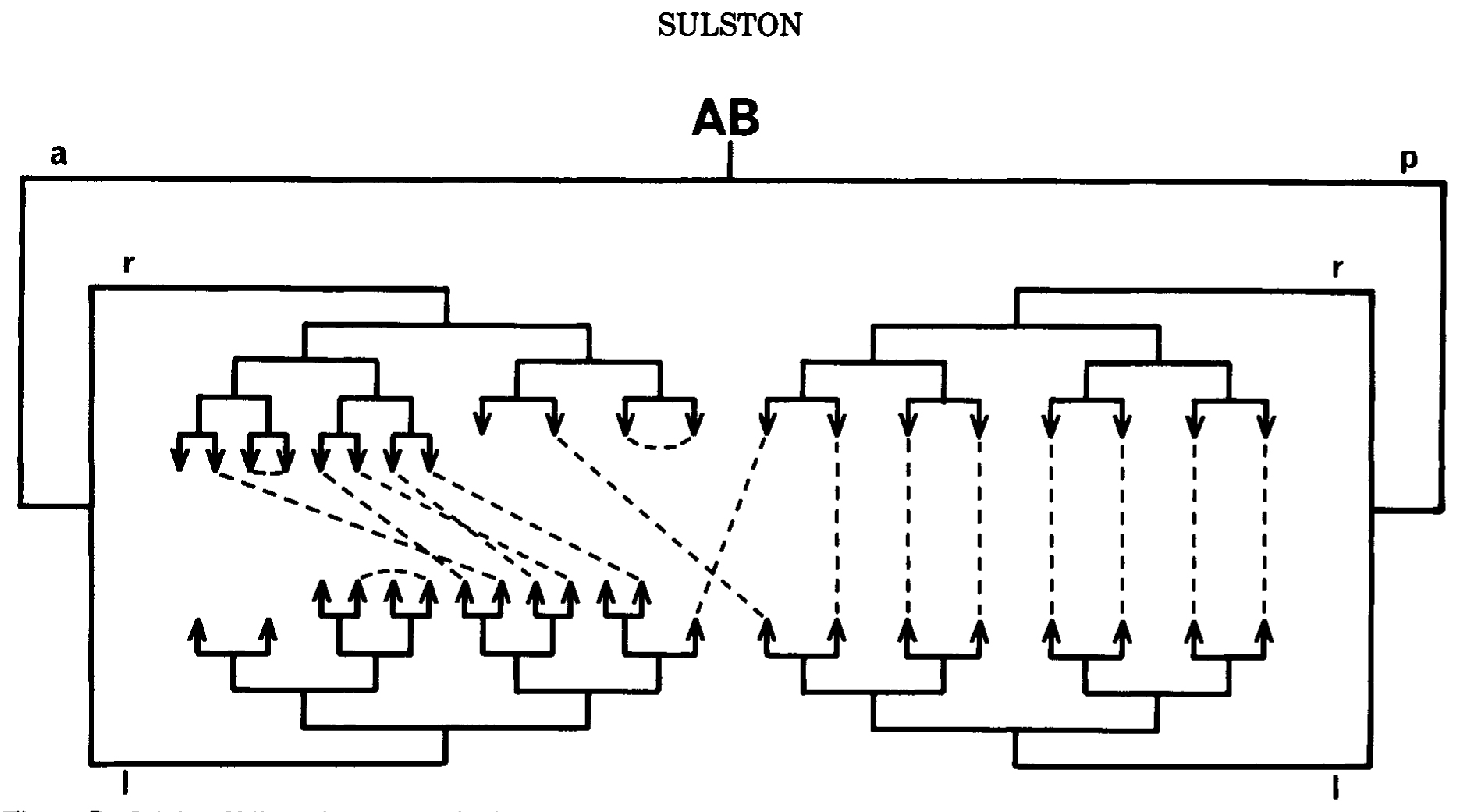
Figure 7. Origin of bilateral symmetry in the embryo. Pairs of precursors that are analogous, in the sense that they genrate approximately the same groups of cells on the left and right sides of the animal, respectively, are linked by dotted lines. The digram shows all such analogs arising in the AB lineage up to the sixth round of cell division (some later ones are shown in FIg.4) ABp gives rise predominantly to homologous analogs, ABa to nonhomologous analogs. I am indebted to Ed Hedgecock for suggesting the form of this figure.
Comparisons between Species
The uniformity of early development in the phylum Nematoda (Chitwood and Chitwood 1974; Sulston et al. 1983) leads to striking cellular homology between the young larvae of different species. Sternberg and Horvitz have exploited this circumstance by following the postembryonic lineages of Panagrellus redivivus for comparison with those of C. elegans. Neurogenesis is very similar in the two nematodes, so that those changes that do occur can be accurately defined (Sternberg and Horvitz 1982).
Of particular revelance to the present discussion is the transformation of a neuron (descended from founder cell C) in C. elegans into a neuroblast in P. redivivus; concomitantly, an adjacent neuroblast in C. elegans is transformed into a neuron in P. redivivus. Since the sublineages generated by the two neuroblasts appear identical, Sternberg and Horvitz have termed this phenomenon (and others like it) "altered segregation"; it is the interspecific equivalent of the production of nonhomologous analogs within a single individual (see above).
Cells Having Multiple Functions
During development. It is not uncommon for a cell to be used in more than one way during development. Usually, but not always, the change of function involves cell division.
In young larvae, most postembryonic neuroblasts are hypodermal cells: That is to say, they underlie the cuticle and probably help to secrete it. When the time comes for neurogenesis to begin, some withdraw fromm the hypodermal layer all together, but the majority divide into hypodermal cell (which remains attached to its neighbors) and a neuroblast (which is budded off inwards). An especially elaborate sequence of events is associated witht he excretory pore, which is initially surrounded by a cell called G1. In the L1 larva, G1 divides into two neurons and its pore-forming function is taken over by another cell, G2. later, G2 divides into a neuroblast and the mature pore cell G2.p (Sulston et al. 1983).
The rectal cell Y provides an example of functional change in the absence of division: Hypodermal in the newly hatched larva, it subsequently moves interiorly and becomes the neuron known as PDA. Sometimes the role of an already differentiated neuron changes, an extreme example being the reversal of information flow in one class of motor neurons (White et al. 1978).
Between sexes. Comparison of the male with the hermaphrodite reveals a variety of cells that are used in alternative ways. Certain larval hypodermal cells (B, F, U, seam cells, preanal ganglion, equivalence group [Fig.5]) that remain hypodermal in the hermaphrodite become neuroblasts in the male. The cells that become VC motor neurons in the hermaphrodite (Fig.3) undergo a further round of division in the maturing male, to yield additional neurons. The behavior of cell Y, already refered to, is intriguing: In the male it withdraws from the hypodermis, as it does in the hermaphrodite, but then divides into a neuron identical with PDA and a male-specific neuroblast.
These examples of cells that function differently in the two sexes are to be contrasted with the mutually exclusive sets of cell deaths by which embryonic sexual dimorphism is achieved (see above).
Conclusion
By way of summary, it will perhaps be helpful to draw together some of these observations in an evolutionary context.
1. In parallel with other elements of biological organization, from genes to limbs, the cell lineage has probably evolved by duplication (cf. Goodman et al. 1977; Chalfie et al. 1981). Duplications have sometimes occured at termini, thereby generating clones of identical cells, but in the lineages giving rise to the nervous system they have more often appeared at earlier points, thereby generating repeated sublineages. Furthermore, since every cell carries the same genetic information, duplications can arise at unrelated points, and so generate nonhomologous analogs.
2. The resulting repetitious elements then seem to have evolved by diversification. In support of this notion are: (a) the high incidence of programmed cell death, pointing to the elimination of specific cells; (b) the relative absence of overt repetition in the embryonic part of the lineage, which is presumably of the greatest antiquity; (c) direct evidence from mutations that affect the lineage (Chalfie et al. 1981; Horvitz, this volume).
3. So perfectly has the segregation of cells by the lineage been matched to the anatomical requirements of the animal that few cells have to move substantially from the places where they are born (discussed further by White, this volume). This matching has been achieved at the expense of order in the cell lineage; we can conclude, therefore, that evolution has proceeded more often by respecifying cells than by moving them about.
4. The generation of nonhomologous analogs, the phenomenon of alteredd segregation, and the lack of cell migration point to cell determination by position rather by ancestry. There are relatively few places where such plasticity can be detected now, but it may well have played an important part in the evolution of the system (Sternberg and Horvitz 1982); and indeed, evidence from the mutant lin-12 points to the continued existence of cryptic equivalence groups (Greenwald et al. 1983; Horvitz et al., this volume).
Although the points made in this summary are speculative at present, they have the merrit of drawing attention to the patterns of control that may operate in the lineage. With the description of the wild type in hand, a number of researchers have turned their attention to mutations that affect the lineage, in the hope of directly identifying the control elements. An account of some of this work is given by Horvitz et al. (this volume).
Acknowledgments
I am greatly indebted to numerous colleagues for sharing their unpublished work and ideas, and in particular to Bob Horvitz and John White for advice on the manuscript.
References
Albertson, D.G. and J.N. Thomson. 1976. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. 275: 299
Bate, M., C.S. Goodman, and N.C. Spitzer.
1981. Embryonic development of identified neurons: Segment-specific differences in the H cell homologues. j. Neurosci. 1: 103.
Chalfie, M., H.R. Horvitz, and J.E. Sulston. 1981. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell 24: 59.
Chalfie, M., J.N. Thomson, and J.E. Sulston. 1983. Induction of neuronal branching in Caenorhabditis elegans. Science 221: 61.
Chitwood, B.G. and M.B. Chitwood. 1974. Introduction to nematology. University Park Press, Baltimore, Maryland.
Goodman, C.S. 1977. Neuron duplications and deletions in locust clones and clutches. Science 197: 1384.
Greenwald, I.S., P.W. Sternberg, and H.R. Horvitz. 1983. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell 34: 435.
Hedgecock, E.M., J.E. Sulston, and J.N. Thomson. 1983. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science 220: 1277.
Horvitz, H.R. 1981. neuronal cell lineages in the nematode Caenorhabditis elegans. In The Fifth Symposium of the British Society for Developmental Biology (ed. Garrod and Feldman), p. 331. Cambridge University Press, Cambridge, England.
Horvitz, H.R., H.M. Ellis. and P.W. Sternberg. 1982a. Programmed cell death in nematode development. Neurosci. Comment. 1: 56.
Horvitz, H.R., M. Chalfie, C. Trent, J.E. Sulston, and P.D. Evans. 1982b. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012.
Jacobson, M. 1978. Developmental neurobiology. Plenum Press, New York.
Kimble, J. 1981. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev. Biol. 87: 286.
Kimble, J. and D. Hirsh. 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396.
Kimble, J.E. and J.G. White. 1981. On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 81: 208.
Laufer, J.S., P. Bazzicalupo, and W.B. Wood. 1980. Segregation of developmental potential in early embryos of Caenorhabditis elegans. Cell 19: 569.
Lawrence, P.A. 1966. Developmental and determination of hairs and bristles in the millkweed bug, Oncopeltus fasciatus (Lygacidae, Hemiptera). J. Cell Sci. 1: 475.
Lawrence, P.A. and S.M. Green. 1979. Cell lineage in the developing retina of Drosophila. Dev. Biol. 71: 142.
Le Douarin, N. 1980. Migration and differantiation of neural crest cells. Curr. Top. Dev. Biol. 16: 31.
Sternberg, P.W. and H.R. Horvitz. 1982. Postembryonic nongonadal cell lineages of the nematode Panagrellus redivivus: Description and comparison with those of Caenorhabditis elegans. Dev. Biol. 93: 181.
Sulston, J.E. and H.R. Horvitz. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56: 110.
Sulston, J.E. and J.G. White. 1980. Regulation and cell autonomy durng postembryonic development of Caenorhabditis elegans. Dev. Biol. 78: 577.
Sulston, J.E., D.G. Albertson, and J.N. Thomson. 1980. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 78: 542.
Sulston, J., M. Dew, and S. brenner. 1975. Dopaminergic neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 163: 215.
Sulston, J.E., E. Schierenberg, J.G. White, and J.N. Thomson. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 98: (in press).
Ward, S., N. Thomson, J.G. White, and S. Brenner. 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160: 313.
Ware, R.W., C. Clark, K. Crossland, and R.L. Russell. 1975. The nerve ring of the nematode Caenorhabditis elegans. J. Comp. Neurol. 162: 71.
White, J.G., D.G. Albertson, and M.A.R. Anness. 1978. Connectivity changes in a class of motoneurone during the development of a nematode. nature 271: 764.
White, J.G., E. Southgate, J.N. Thomson, and S. Brenner. 1976. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. 275:327.
|