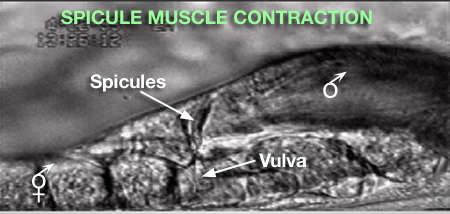
2 Spicule Neuron Connectivity
Major synaptic targets of the spicule neurons include each other, hook and PCS neurons, the protractor muscles and the gonad (The Male Wiring Project). These connections emphasize that spicule behavior must be coordinated with the preceding step of vulval location and the subsequent step of sperm transfer (reviewed in WormBook: Male Mating Behavior - Barr and Garcia, 2006). During mating the hook and PCS detect the general location of the vulva and trigger spicule prodding behavior by inducing periodic contraction of the spicule protractor muscles (MaleSpicMOVIE 1). This stimulation appears to be indirect as neither hook nor PCS neurons synapse onto the muscles. SPCL/R, having both proprioceptive and motor connections with the protractor muscles, register when the vulval slit is breached and induce tonic muscle contraction and full spicule insertion (MaleSpicFIG 6B; MaleSpicFIG 7B; Garcia et al, 2001). Sensory neurons SPVL/R are required to inhibit premature ejaculation (Liu and Sternberg, 1995) but surprisingly do not have direct connections to the gonad. These neurons may therefore exert their effect through their connections with SPCL/R and the PCS neurons which innervate vas deferens and cloacal cells in the gonad-cloacal junctional region (MaleSpicFIG 7C). SPDL/R connectivity are not known however they are the major targets of SPVL/R.
MaleSpicFIG 7A: Spicule muscles. Diagram of location of spicule muscles and SPC neuron, lateral view. (PAG) Pre-anal ganglion; (NMJ) neuromuscular junction.
MaleSpicFIG 7B&C: Synapses in the spicules. B. TEM of region indicated in 7A showing synapse between SPC and protractor muscles, transverse view. (Image source: N2Y [MRC] 1221.) C. TEM of region indicated in 7A showing synapse between PCC and the vas deferens, transverse view. (Image source: N2Y [MRC] PAG 875.)
3 Spicule Development
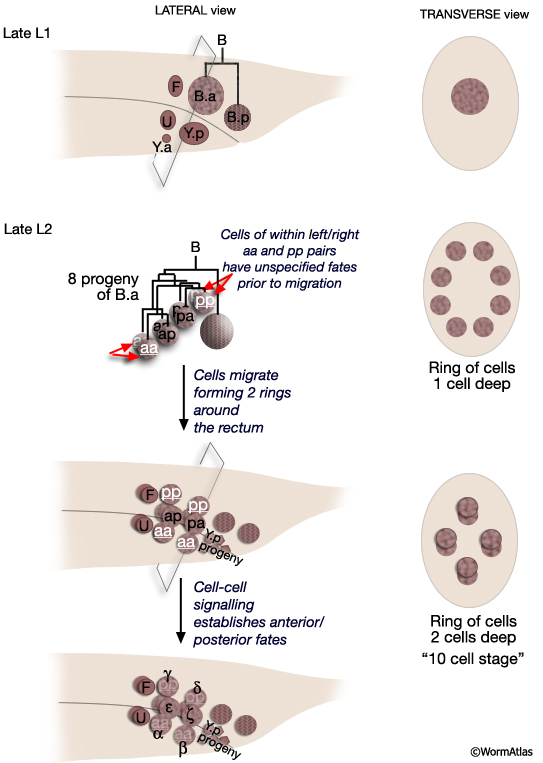
All spicule cells, including spicule neurons, are derived from the rectal cell B which is present in the L1 of both sexes but is a blast cell only in the male (MaleSpicFIG 8; Sulston and Horvitz, 1977; Sulston et al., 1980). In males, B generates 47 progeny which form the cloaca and spicule channels that house the spicules, as well as the spicules themselves. Spicule development can be divided into 2 phases (1) spicule cell generation and (2) spicule morphogenesis.
MaleSpicFIG 8: Lineal origin of spicule cells. **α vs β and γ vs δ fates determined by positional cues (see MaleSpicFIG 9). Socket cells (SPso1-4L/R) of the L/R side fuse forming a 4-cell syncytium. Sheath cells (SPshD/V L/R) of the L/R side fuse forming a 2-cell syncytium (see MaleSpicTABLE 1). *Spicule neurons. (Adapted from Sulston et al., 1980; Chamberlin and Sternberg, 1994; Jiang and Sternberg, 1999.)
3.1 Spicule cell generation
The B cell lineage is defined by three distinct phases: early division, short range migrations and late divisions (MaleSpicFIG 8). The early divisions begin in late L1 where the B cell divides asymmetrically to produce a large anterior daughter (B.a) and a small posterior daughter (B.p). This asymmetry is regulated by the Wnt pathway (a LIN-44 /LIN-17 pathway; Sternberg and Horvitz, 1988; Herman and Horvitz, 1994). By mid-L2 B.a and B.p produce a total of 10 cells (MaleSpicFIG 8, MaleSpicFIG 9): B.a generates 4 cell pairs and B.p 1 pair. The 4 B.a-derived pairs migrate a short distance, arranging themselves in 2 rings of 4 cells around the developing cloaca. Among the aa and pp cells migration of the left and right cell is variable; either the left or right member can take the anterior position near the cloaca. Signals from neighboring cells of the F, U (EGF LIN-3/ LET-23 signaling) and Y lineages (LIN-15) promote differences between these otherwise equivalent cells causing them adopt an anterior fate (for aa's = α, for pp's=γ) or a posterior fate (for aa's = β; for pp's= δ) (distinguished by the lineage produced in later cell divisions; Chamberlin and Sternberg, 1993; 1994). Thus, the B lineage represents one of the few cases in C. elegans where cell fate is not fixed by lineal origin and is determined by environmental cues. Cells of the B.p lineage are patterned by Notch signaling (LIN-12) (MaleSpicFIG 8; Chamberlin and Sternberg, 1994). During L3, precursors α, β, ζ and γ generate the cells of the spicules.
3.2 Spicule morphogenesis
The B cell lineage is complete by the L3/L4 molt. Spicule morphogenesis occurs during L4 and is part of a larger process of tail remodeling that begins at mid-L4 (38-40hrs, 20°C) and ends by the final molt (45hrs) (MaleSpicFIG 9; MaleSpicFIG 10; MaleSpicFIG 11; MaleSpicFIG 12); for tail remodeling see Epithelial System of the Male - Hypodermis). In the adult, 2 pairs of proctodeal cells, Bε(l/r).pv (aka B.a(l/r)appv) and Bε(l/r).apa (B.a(l/r)apapa), lie immediately dorsal and ventral of the spicules and line the spicule channels (MaleSpicFIG 10). These cells play a major role in establishing the characteristic elongated morphology of the spicules and the channel. During morphogenesis Bε(l/r).pv and Bε(l/r).apa migrate anteriorly, establishing cellular molds (spicule traces) into which future spicule cuticle is secreted (MaleSpicFIG 10). Their migration is dependent on a TGF-β (DBL-1) signal, possibly coming from dorsal sex muscles (Baird and Ellazar, 1999).
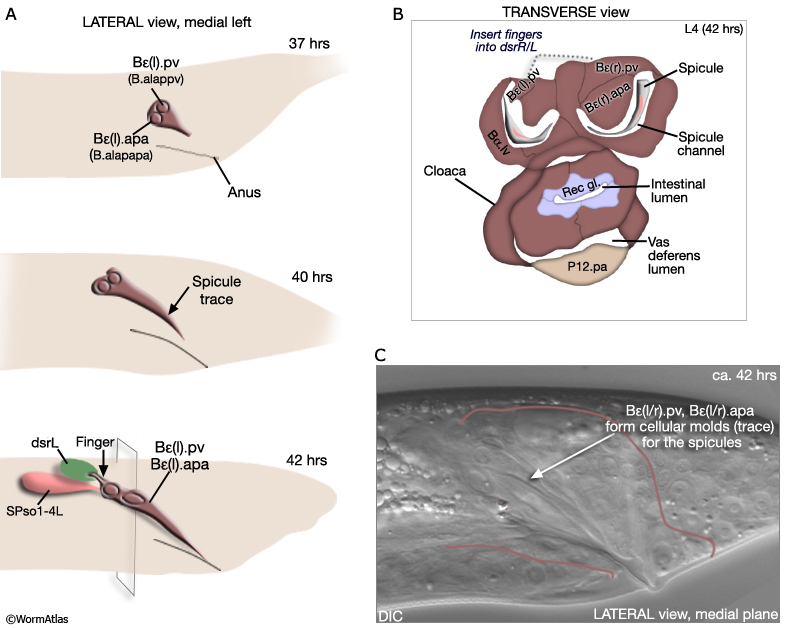 Bε(l/r).pv insert "fingers" into the dorsal spicule retractor (dsr) muscles (MaleSpicFIG 10; see also MaleProcFIG 7 in Epithelial System of the male - Proctodeum). The forward movement of retractors during tail morphogenesis likely facilitates spicule trace elongation. An FGF signal (EGL-17), expressed in the SPso cells (MaleSpicFIG 11), has also been implicated in the process (Jiang and Sternberg, 1999; reviewed in WormBook: Male Development - Emmons). The SPso cells are essential for spicule elongation and also for secretion of the spicule cuticle, which begins at ca. 42 hrs (Jiang and Sternberg, 1999). Both spicule elongation and cuticle formation depend on the activity of heterochronic transcription factor LIN-29 which is expressed in B lineage cells (Euling et al., 1999).
MaleSpicFIG 11: Spicule trace elongation. A. Nomarski DIC image of late L4 male tail formation showing location of SPso cell body, lateral view, left sublateral plane. B. Transgenic animals expressing the egl-17::GFP reporter gene in male tail. (Strain source: R.D. Burdine and M.J. Stern.) (Cell identification by L.I. Jiang and P.W. Sternberg.)
MaleSpicFIG 12: Ultrastructure of the developing spicule. A. TEM of late L4 stage male featuring the spicules, transverse view. (Image source: JSG [MRC] 270.) B. Detailed view of boxed region in A featuring the adherens junctions (AJs) between the proctodeal cells and spicule processes.
4 References
Baird, S.E. and Ellazar, S.A. 1999. TGFbeta-like signaling and spicule development in Caenorhabditis elegans. Dev. Biol. 212: 93-100. Article
Chamberlin, H.M. and Sternberg, P.W. 1993. Multiple cell interactions are required for fate specification during male spicule development in Caenorhabditis elegans. Development 188: 297-324. Article
Chamberlin, H.M. and Sternberg, P.W. 1994. The lin-3/let-23 pathway mediates inductive signalling during male spicule development in Caenorhabditis elegans. 120: 2713-21. Article
Emmons S.W. and Sternberg, P.W. 1997. Male Development and Mating Behavior. In C. elegans II (ed. D. L. Riddle et al.). Chapter 12. pp.295-334. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Article
Euling, S., Bettinger, J.C. and Rougvie, A.E. 1999. TGFbeta-like signaling and spicule development in Caenorhabditis elegans. Dev. Biol. 206: 142-56. Article
Garcia, L.R., Mehta, P. and Sternberg, P.W. 2001. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell 107: 777-788. Article
Herman, M.A. and Horvitz, H.R. 1994. The Caenorhabditis elegans gene lin-44 controls the polarity of asymmetric cell divisions. Development 120: 1035-47. Article
Jiang, L.I. and Sternberg, P.W. 1999. Socket cells mediate spicule morphogenesis in Caenorhabditis elegans males. Dev. Biol. 211: 88-99. Article
Liu, K.S. and Sternberg, P.W. 1995. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14: 79-89. Article
Sternberg, P.W. and Horvitz, H.R. 1988. lin-17 mutations of Caenorhabditis elegans disrupt certain asymmetric cell divisions. Dev. Biol. 130: 67-73. Abstract
Sulston, J.E. and Horvitz, H. R. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56: 110-156. Article
Sulston, J.E., Albertson, D.G. and Thomson, J.N. 1980. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev Biol. 78: 542-576. Article
Sulston, J.E., Schierenberg, E., White J.G. and Thomson, J.N. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64-119. Article
|