EmbryoIntroFIG 1: Embryonic stages of development. Numbers below the horizontal axis show approximate time in minutes after fertilization at 20-22°C. First cleavage occurs approximately 40 minutes after fertilization. (Yellow-green bars) Period of time during which cells from a certain lineage migrate towards inside of the embryo. The first cells that move inwards from the ventral surface are gut precursors (E), followed by mesoderm (MS) and germline (P4) precursors. (Blue bar) Gastrulation. Gastrulation cleft is closed by short-range movement of ectodermal cells (neuroblasts, postmitotic neurons, glia and glia precursors) between 270 and 330 minutes (Chin-Sang and Chisholm, 2000). (Red bar) Elongation of the embryo that occurs between 400 and 640 minutes due to circumferential contraction within the hypodermis. During elongation, the embryo becomes threefold thinner and its length increases about fourfold. Sexual dimorphism becomes visible for the first time at 510 min when the cephalic companion neurons (CEM) die in the hermaphrodite, whereas the hermaphrodite-specific neurons (HSN) die in the male. The stages, number of nuclei, marker events and DIC images of the embryos and a newly hatched larva are shown above the horizontal axis. (Based on von Ehrenstein and Schierenberg, 1980; Sulston et al., 1983; Wood, 1988; Bucher and Seydoux, 1994; Chin-Sang and Chisholm, 2000.)
1 Fertilization
Immediately after fusion of an oocyte and a spermatozoon within the spermatheca, the diploid cell displays a new covering, perhaps combining elements from the zona pellucida with exocytosis from intracellular stores of glycoprotein from the oocyte (EmbryoIntroFIG 2) (Foor, 1967; Weidman et al., 1985). Cortical granules are released coincident with fertilization, and likely contribute to the formation of a fertilization membrane and/or the eggshell, or both (Bembenek et al., 2007). TEM evidence for an intact fertilization membrane has been obtained by high pressure freeze fixations of normal hermaphrodite adults (Hall and Greenstein, unpublished; EmbryoIntroFIG 2). This glycocalyx is also quickly removed, probably within a few minutes, as the new embryo begins to produce an eggshell underneath it. The glycocalyx breaks away into the uterine lumen and is probably dissolved or expelled from the lumen during egg-laying. Rapid production of a fertilization membrane can offer a physical block to fertilization by more than one sperm until the eggshell is in place. The eggshell rapidly replaces the fertilization membrane, and is made up of a completely different set of components (Bembenek et al., 2007; Olson et al., 2012). For more details see Oegema and Hyman, 2006 and Stein and Golden, 2015 in WormBook.
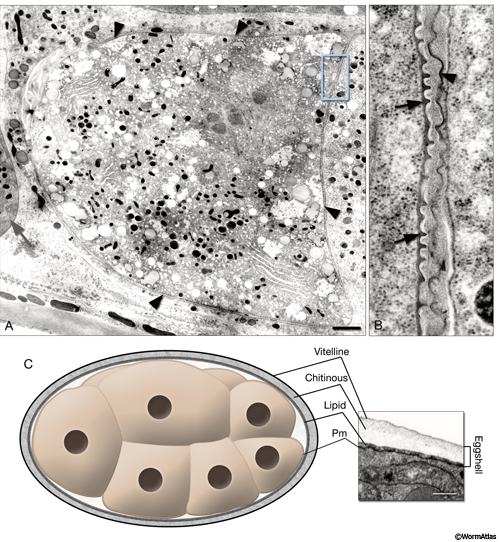 EmbryoIntroFIG 2: Fertilization membrane and eggshell. A. For a brief period, a recently fertilized embryo becomes covered with a dense, crenellated membrane (arrowheads) that may prevent polyspermy. (Gray arrow) Spermatheca. TEM-high pressure freeze fixation, transverse section. Bar, 1 µm (Image source: [Hall] CL2099-2A.) B. Same image as in A, magnified. The fertilization membrane of the embryo on the left (arrows) is still attached to its surface whereas that of an older embryo on the right is being sloughed off (arrowhead). C. Graphic rendition of the eggshell of an embryo. The eggshell consists of three layers secreted by the egg itself: an outer vitelline layer, a middle chitinous layer, and an inner lipid-rich layer. (Pm) Plasma membrane. The inset on the right is a TEM image of an eggshell. Laser hole fixation. Bar 0.3 µm. (Image source: [Hall] N611-565.)
2 Proliferation and Gastrulation
Proliferation (0 to 330-350 min post-fertilization at 22°C) includes cell divisions from a single cell to about 550 essentially undifferentiated cells by the end of the “16 E stage” (von Ehrenstein and Schierenberg, 1980; Wood, 1988). This stage is further subdivided into two phases: The first phase (0-150 min) spans the time between zygote formation to generation of embryonic founder cells (see EmbryoIntroVID 2), and the second phase (150-350 min) covers the bulk of cell divisions and gastrulation until the beginning of organogenesis (Bucher and Seydoux, 1994) (EmbryoIntroFIG 1, EmbryoIntroFIG 3, EmbryoIntroVID 3 & EmbryoIntroVID 4).
EmbryoIntroVID 2: Proliferation. Nomarski DIC video showing development of a C. elegans embryo from the first cell division until hatching. Video pauses briefly at several key times, such as when founder cells are born. Embryo raised at 20°C. (Movie source: B. Goldstein.)
EmbryoIntroFIG 3: Life cycle of C. elegans at 22°C. Fertilization occurs at -50 min. Numbers along the bottom of the arc indicate the time period the animal spends at a certain stage, where "zero time" is the first cleavage event. Eggs are laid outside the mother at about 150 min.
The initial 150 min of proliferation takes place within the mother’s uterus, and the embryo is laid outside when it reaches the approximate 30-cell stage (at the start of gastrulation). Depending on the rate at which the hermaphrodite mother is producing fertilized eggs, each growing embryo may reside inside the uterus for several hours, and progress from the 1-cell stage to about the 16 cell or 32 cell stage prior to egg-laying (EmbryoIntroFIG 4). The hermaphrodite mother continues to produce more fertilized embryos for several days until she runs out of available sperm. As she reaches about 5 days of adulthood, the mother’s rate of embryo creation slows down, and fertilized embryos may reside within her uterus even longer, and achieve more mature status before the mother can lay them. Mating of the hermaphrodite with a fertile male can provide more sperm, extend the time of her fertility, and increase the total number of viable progeny produced.
There is considerable rearrangement of cells in the proliferation stage because of short-range shuffling, and once gastrulation begins, because of specific cell migrations (during gastrulation Ea and Ep sink in from the posterior and enter into the embryo at 100 min after first cell cleavage. P4 and MS progeny enter at 120-200 min, followed by C and D myoblasts entering from the posterior. AB-derived pharynx progenitors enter inside at 210-250 min. The process through which cells move away from their place of origin within the early embryo has been termed global cell sorting (Schnabel et al., 2006; Bischoff and Schnabel, 2006). Once the cell migrations are completed, the ventral cleft through which cells migrated inward, closes proceeding from the posterior (230 min) to anterior. Ventral cleft closure is completed at 290 min). These events are shown in EmbryoIntroVID 3 and EmbryoIntroVID 4.
From this time onward, the embryonic substages can be defined by specific cell migrations, gain in cell number, and periods of synchronous stem-cell divisions. For more detail see Nance et al., 2005 in WormBook. During these earliest times in proliferation, all cells remain relatively undifferentiated and undergo complete cell divisions, although the presumptive germline precursor cell P4 already contains unique P granules (Updike and Strome, 2010; Updike et al., 2014; Wang and Seydoux, 2014). In this early stage, prior to morphogenesis, all embryonic cells establish early contacts with nearest neighbors via INX-3 gap junctions (Starich et al., 2003), but there are not yet any syncytial tissues.
EmbryoIntroVID 3: Ventral view of gastrulating C. elegans embryo. DIC video showing Ea and Ep moving towards center of embryo as their neighbors surround them. Video pauses briefly at several key times to indicate specific cells. Ea and Ep will then be the first cells to move inward to begin gastrulation itself. Founder cell names follow Sulston et al, 1983 (see also EmbryoIntroFIG 6). (Movie source: Lee and Goldstein, 2003, hosted with permission.)
EmbryoIntroVID 4: Labeled gastrulation events in the embryo. Four-part movie. Upper left shows the raw video of a plasma membrane-tagged embryo; upper right outlines the cell boundaries; lower left marks gastrulating cells with colors; lower right provides renderings of cell outlines as generated from a membrane-marked embryo filmed by spinning disk confocal microscopy at a plane corresponding to the middle of the top layer of cells at each stage, i.e. halfway through the depth at the four cell stage, and rising to match the middle of the layer of cells nearest the objective lens as cell divisions resulted in smaller and smaller cells. View is initially a lateral view, becoming a ventral view as the embryo rotates after E lineage (green) internalization. E, MS, P4, D and all of their descendants are colored from the time they are born. In the AB and C lineages, only some descendants gastrulate. Colored cells in bottom panels seem to disappear along a ventral furrow at the moment that they gastrulate, each moving inward to the center of the embryo, thus becoming out of view from this aspect For these lineages, coloring is as follows: the AB cells (in purple) only during the cell cycle at which each cell internalization occurs, the C lineage (in yellow) at the birth of C, with yellow later marking only those C lineage cells that internalize. 50 of the 66 gastrulating cells are shown here. The remaining 16 gastrulating cells (all from the AB lineage) internalize from a site other than the ventral side of the embryo. Frames were acquired 1 min apart. (Movie source: J. Iwasa and J. Harrell from Harrell and Goldstein, 2011, hosted with permission.)
3 Morphogenesis
During the organogenesis/morphogenesis stage (5.5-6 hr to 12-14 hr), terminal differentiation of cells occurs without many additional cell divisions, and the embryo elongates threefold and takes form as an animal with fully differentiated tissues and organs. Morphogenesis starts with the “lima bean” stage. The first muscle twitches are observed at 430 min after the first cell cleavage (between 1.5- and 2-fold stages) (EmbryoIntroFIG 1; EmbryoIntroVID 1). This likely represents spontaneous muscle activity, as synaptic connections from the nervous system are still not present or are incomplete. Sexual dimorphism becomes visible for the first time at 510 minutes when the cephalic companion neurons (CEMs) die in the hermaphrodite, and when the hermaphrodite-specific neurons (HSNs) die in the male (see MaleIntroFIG 6). In the late three-fold stage, the worm can move inside the egg in a coordinated fashion (rolling around its longitudinal axis), indicating advanced motor system development. C. elegans initiates pharyngeal pumping at 760 min after the first cell cleavage and hatches at 800 min (von Ehrenstein and Schierenberg, 1980; Sulston et al., 1983; Bird and Bird, 1991). For greater detail see Chisholm and Hardin, 2005 in WormBook.
4 Elongation
Following epidermal enclosure (350-390 minutes), the process of elongation converts the worm from a "bean" shape into a longer, thinner animal. This process begins around 400 minutes and continues through the comma stage, 1.5-fold stage and to the 3-fold stage (EmbryoIntroVID 5) (see also EmbryoIntroVID 1). Later, during the quickening phase, periodic circumferential squeezing of the body helps to further transform the body into a longer, slimmer profile. For greater detail see Chisholm and Hardin, 2005 in WormBook.
EmbryoIntroVID 5: Elongation in the embryo. Nomarski video of embryo undergoing elongation. Frames were collected every minute for 90-110 min (8 frame/s display rate). (Movie source: Pettitt et al., 2003, hosted with permission.)
5 Quickening
In the late three-fold stage, the worm can move inside the egg in a coordinated fashion (rolling around its longitudinal axis), indicating advanced motor system development is now being directed by nervous system input (see EmbryoIntroVID 1 and EmbryoIntroVID 6). C. elegans initiates pharyngeal pumping at 760 min after the first cell cleavage and hatches at 800 min (von Ehrenstein and Schierenberg, 1980; Sulston et al., 1983; Bird and Bird, 1991). For greater detail see Chisholm and Hardin, 2005 in WormBook.
6 Hatching
EmbryoIntroVID 6 captures the moments just prior to and includes the moment of hatching (see also EmbryoIntroVID 1). It is unclear what happens just prior to hatching that may weaken the eggshell locally as there is no obvious change in the anatomy of the animal at this time that is indicative it is now ready for hatching. Perhaps a pharyngeal secretion of a chitinase facilitates this process? One can detect a local deformation (weakening) of the eggshell at the nose of the animal on the left in EmbryoIntroVID 6, just prior to hatching. Currently, hatching enzymes are better characterized for parasitic nematodes than for C. elegans (Barrett, 1976; Perry et al., 1991; Wharton, 1986), but parasitic nematodes may hatch according to different (external) stimuli, and some species progress to a more advanced larval stage inside the eggshell prior to hatching. Once freed from its eggshell, the animal stops rolling motions and immediately shows local searching motions of the head, as the animal haltingly moves forward. Now the pharynx can begin pumping food into the nematode, and the animals body can start to increase in total volume for the first time.
EmbryoIntroVID 6: Hatching. Nomarski DIC video of embryos hatching. (Movie source: P. Partensky, Woods Hole Marine Biological Labs, 2006; hosted with permission.)
7 Embryonic Cell Lineage
The entire cell lineage of the C. elegans embryo has been tracked from fertilization through hatching by Sulston et al., 1983 and generates 671 cells, 113 of which undergo cell death in the hermaphrodite (111 in the male). At the time of hatching, the hermaphrodite worm has 222 neurons (202 somatic, 20 pharyngeal) and most of these derive from the AB lineage (the male worm has 224 neurons at this stage).
EmbryoIntroFIG 5: Embryonic cell lineage. Vertical axis represents time at 20C, from 0 min at first cleavage to 800 min at hatching. Many of the observations were made on eggs which were developing at slightly different rates (due to temperature variation and the effect of prolonged illumination); these primary results were normalised, by means of certain prominent cell divisions, to the course of events in eggs which were kept at 20C and viewed infrequently. The precise times of individual events were not our primary concern, and should not be taken too seriously; the likely error varies from 10% at the beginning of the lineage to 2% at 400 min. Horizontal axis represents the direction of cell division. Each terminal branch of the embryonic lineage is labelled either with X (indicating cell death; the position of the X on the time axis indicates the time of maximum refractility) or with a lineage name followed by a functional name. Large arrowheads denote cells which divide postembryonically, and small arrowheads denote nuclei which divide postembryonically, Symbols O and --- link precursors which give rise to bilaterally symmetrical groups of cells, the symbol ~ being included for cases of imperfect symmetry, cord, ventral cord; gang, ganglion; lumb, lumbar; d-r, dorsorectal; p-a, preanal, r-v, retrovesicular; lat neur, isolated neuron lying laterally.
8 References
Barrett, J. 1976. Studies on the induction of permeability in Ascaris lumbricoides eggs. Parasitology 73: 109-21. Abstract
Bembenek, J.N., Richie, C.T., Squirrell, J.M., Campbell, J.M., Eliceiri, K.W., Poteryaev, D., Spang, A., Golden, A. and White, J.G. 2007. Cortical granule exocytosis in C. elegans is regulated by cell cycle components including separase. Development 134: 3837-48. Article
Bird A.F. and Bird J. 1991. The structure of nematodes. Academic Press, California.
Bischoff, M and Schnabel, R. 2006. A Posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLOS Biology doi.org/10.1371/journal.pbio.0040396 Article
Bucher, E.A. and Seydoux, G. 1994. Gastrulation in the nematode Caenorhabditis elegans. Sem. Dev. Biol. 5: 121-130. Abstract
Chin-Sang I.D. and Chisholm, A.D. 2000. Form of the worm: genetics of epidermal morphogenesis in C. elegans. Trends in Genetics 16: 544-551. Abstract
Chisholm, A.D. and Hardin, J. 2005. Epidermal morphogenesis. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.35.1. Article
Foor, W.E. 1967. Ultrastructural aspects of oocyte development and shell formation in Ascaris lumbricoides. J. Parasitol. 53: 12451261. Abstract
Harrell, J.R. and Goldstein, B. 2011. Internalization of multiple cells during C. elegans gastrulation depends on common cytoskeletal mechanisms but different cell polarity and cell fate regulators. Dev. Biol. 350: 1-12. Article
Lee, J-Y. and Goldstein, B. 2003. Mechanisms of cell positioning during C. elegans gastrulation. Development 130: 307-320. Article
Nance, J., Lee, J-Y. and Goldstein, B. 2005. Gastrulation in C. elegans. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.23.1. Article
Oegema, K. and Hyman, A.A. 2006. Cell division. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.72.1. Article
Olson, S.K., Greenan, G., Desai, A., Müller-Reichert, T. and Oegema, K. 2012. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J. Cell Biol. 198: 731-748. Article
Perry, R.N., Knox, D.P. and Beane, J. 1992. Enzymes released during hatching of Globodera rostochiensis and Meloidogyne incognita. Fundam. Appl. Nematol. 15: 283-88. Article
Petttitt, J. Cox, E.A., Broadbent, I.A., Flett, A. and Hardin, J. 2003. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherincatenin function during epidermal morphogenesis. J. Cell Biol. 162: 15. Article
Schnabel, R., Bischoff, M., Hintze, A., Schulz, A-K., Hejnol, A., Meinhardt, H. and Hutter, H. 2006. Global cell sorting in the C. elegans embryo defines a new mechanism for pattern formation. Dev. Biol. 294: 418-31. Article
Starich, T., Miller, A., Nguyen, R.L., Hall, D.H. and Shaw, J. 2003. The C. elegans innexin gene product INX-3 is localized to gap junctions and is essential for embryonic development. Dev. Biol. 256: 403-417. Article
Stein K.K. and Golden A. 2015. The C. elegans eggshell. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.179.1. Article
Sulston, J.E., Schierenberg, E., White J.G. and Thomson, J.N. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64-119. Article
Updike, D. and Strome, S. 2010. P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31: 53-60. Article
Updike D.L., Knutson A.K., Egelhofer T.A., Campbell A.C. and Strome S. 2014. Germ-granule components prevent somatic development in the C. elegans germline. Current Biol. 24: 1-6. Article
Von Ehrenstein, G. and Schierenberg, E. 1980. Cell lineages and development of Caenorhabditis elegans and other nematodes. In Nematodes as biological models Vol. I, Behavioral and developmental models (ed. Zuckerman, B.M.). Chapter 1. pp 2-68. Academic Press, New York.
Wang, J.T. and Seydoux, G. 2014. P granules. Current Biol. 24: R637-38. Article
Weidman, P.J., Kay, E.S. and Shapiro, B.M. 1985. Assembly of the sea urchin fertilization membrane: Isolation of proteoliaisin, a calcium-dependent binding protein. J. Cell Biol. 100: 938-946. Article
Wharton, D.A. 1986. A functional biology of nematodes. Croom Helm, London & Sydney. Abstract
Wood, W.B. 1988. Embryology. In The nematode C. elegans (ed. W.B. Wood). Chapter 8. pp 215-241. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Abstract |
