|
The Nerve Ring of the Nematode
Caenorhabditis elegans:
Sensory Input and Motor Output
Randle W. Ware, David Clark, Kathryn Crossland And Richard L. Russell
Division of Biology, California Institute of Technology, Pasadena, California 91125
J Comp. Neurol (1975) 162: 71-110
DOI: 10.1002/cne.901620106
*New nomenclature equivalents for this paper*
Abstract -
Material & Methods -
Observations -
Discussion -
Acknowledgments
Abstract
The general organization and structure of the nerve ring, the main mass of central nervous system neuropil, in the small soil nematode Caenorhabditis elegans is described. The nerve ring receives sensory input from the anterior tip of the animal by means of six nerve bundles, all nerve fibers of which have centrally located cell bodies. The anterior sensory structures are classically divided into two types, papilIary and amphidial, and are assumed responsible for mechano- and chemoreception, respectively. Papillary fibers enter dIrectly into the nerve ring, whereas amphidial fibers enter the ventral ganglion, a posterior extension of the nerve ring, in a circuitous, manner which is not discussed in detail. Of those papillary fibers which project into the nerve ring neuropil, 22 end in easily characterized sensory structures whereas 14 terminate distally near sensory organs but have no function which can be deduced on the basis of comparative morphology. After entering the ring the fiber, maintain their identity and do not anastamose with one another. Cell bodies of each papillary sensory neuron have been mapped around the nerve ring. The cephalic musculature is shown to consist of 32 muscle cells which form four longitudinal submedial groups of eight muscles each. Innervation of this musculature occurs wholly within the CNS by means of processes of the muscle cells which are sent centrally. The anterior 16 cephalic muscle cells are innervated by the ring only, in well delimited regions termed muscle plates. The posterior 16 are dually innervated by means of processes sent both to the nerve ring plates and to theIr nearest medial longtitudinal nerve cord. The nerve ring neuropil is characterized as having fibers containing one of four morphologically distinct vesicle types. Gap junction contacts are observed within the main neuropil involving one of these fiber types and within the muscle plate regions among muscle processes, which do not contain vesicles. An evolutionarily primitive sensory-motor synapse within the nerve ring is described from an identified sensory neuron onto an identified cephalic muscle cell process. Comparisons are made with the nervous system of Ascaris lumbricoides, the only other nematode to be extensively studied, to ilIustrate the conservativeness of the nemic nervous system.
A number of different approaches have been used in attempts to elucidate the nature of the rules of growth which lead to the observed high degree of specificity of neuronal connections. The most dIrect is simply to follow the growth of nerve fibers during embryogenesis (Waddington and Perry, '69; Hanson, '72; Trujillo-Cenoz and Melamed, '73; LoPresti et al., '73). Inferences have also been derived from neurophysiological investigations of connectivities in adult animals following regeneration of severed nerves or after surgical intervention during development (Sperry, '65; Jacobsen, '70; Yoon, '73) and by characterizing irregularities in the special case of a regular repeated adult structure (Horridge and Meinertzhagen, '70; Meinertzhagen, '72). It has been proposed (Benzer, '67) that the analysis of animals with single gene mutations leading to behavioral alterations can serve this same purpose, with the additional advantage that there is the possibility of analyzing the nature of the mutation biochemically.
Study of nematodes has appeared promising to a number of workers because, in spite of forming an extraordinarily widespread class adapted to live in many varied ecological niches, their varied structures represent fundamentally small variations on the same overall scheme. The central nervous system, in particular, consists of approximately the same number of cells in roughly the same arrangements from the smallest free living forms (less than 1 mm in length) to the very large mammalian parasites (up to 50 cm in length). This similarity may well prove to be useful in comparing the ultrastructure of some members of the group, necessarily the smaller ones, with electrophysiological results obtained from the larger ones.
The small soil nematode C. elegans is an excellent candidate for both mutational and ultrastructural studies. Extensive genetic analyses involving this organism have recently been reported by Brenner ('74) and have been related to DNA content by Sulston and Brenner ('74). Anatomical alterations in mutants have also been described (Brenner, '73). Mutational studies involving chemotactic behavior have been reported by Dusenbery ('73, '74), Ward ('73j and Dusenbery et al. ('75) indicating that mutants with quantifiable behavioral abnormalities can be obtained. Of particular interest for the work reported here, the central nervous system, consisting of about 200 cells, is sufficiently small to enable extensive reconstruction at the level of the electron microscope with an acceptable amount of effort. Because the number of neurons is small and, according to our results, the presence of each is invariant, it is likely that the proper connectivities of everyone is essential for the normal functioning of the animal. Thus one can hope to correlate an observed behavioral alteration with a particular morphologicai alteration in the CNS. In addition, it may be possible to use the analysis of mutants exhibiting partial expressivity to gain some insight into the mechanism of fiber growth and recognition which, in the normal animal produces a stereotyped nervous system with nearly 100% reproducibility.
We report here some results of the necessary first step in using the mutational approach to the study of neurogenesis, that is, the determination of the fundamental features of the wild type structure. These features will be used as a basis for the analysis of our existing chemotaxis, thermotaxis, and head muscle-defective mutants. In what follows we will discuss (i) the fine structure of the various anterior sensory organs and their projections to the central nervous system; (ii) the locations of the anterior sensory cell neurons around the nerve ring, the main part of the central nervous system, as revealed by complete three-dimensional reconstruction; (iii) the general layout and innervation of the cephalic musculature; (iv) the general structure of the neuropil of the nerve ring.
Materials & Methods
C. elegans were cultured on petri plates as described by Dusenbery ('73). For fixation animals which had just reached the egg laying stage were rinsed from plates of synchronized animals, anesthetized for ten minutes with 1% propylene phenoxetol fixed for one hour at room temperature in 2% OsO4 in 0.1 M cacodylate buffer, pH 7.4, sectioned just posterior to the end of the oesophageal musculature, stained for one hour in 1% uranyl acetate in 0.1 M maleate buffer, pH 5.9, and embedded in epon. A persistent problem due to the toughness of the cuticle has been incomplete penetration of the resin except near the cut end. For microtomy individual heads were cut out of the flat mold and attached with epoxy to supportive blocks. Serial sections were cut on a Sorvall MT2B ultramicrotome at thicknesses appropriate to the complexity of the structures being examined, 120 nm where the axes of nerve fiber bundles being followed for long distances were perpendicular to the plane of the section, 50 nm in the CNS where fibers are circumferential and were therefore cut more nearly parallel to theIr axes. The procedure was systematized so that the cutting rate was regularly 300 sections per hour. Ribbons were collected on slot grids covered with carbon coated formvar and stained five minutes in lead citrate No more than 1 or 2% of the sections were lost. Electron microscopy was carried out using a Zeiss EM9 for which a camera capable of holding a 100-foot roll of 70 mm film was built to expedite the making of the approximately 15,000 low magnification electron micrographs used in this study. All micrographs were made on Rekordak microfilm.
Since the nerve and muscle processes discussed look very much alike except at their terminal specializations and intracelluiar staining techniques are impractical in such a small animal, there is no possibility of linking central with peripheral processes except by extensive three-dimensional reconstruction from serial sections. That is, each process must be followed in its entirety through serial elctron micrographs in order to describe it correctly. For convenience in accomplishing this we used an electron microscope serial section cinematographic procedure similar to that first described by Levinthal and Ware ('72). For this procedure an apparatus, shown in figure 1, was constructed which enables micrographs of sequential sections to be aligned and photographed with a 35 mm movie camera. With this apparatus 35 mm movies of up to 800 micrographs of sequential sections have been created on microfilm at a rate of 60- 80 frames per hour In order to make full use of the inherent resolution and contrast range of the microfilm, movies were used in a Vanguard M35 projector which had been modified so that the film plane could be viewed directly with a room dissecting microscope. All results presented derive from this cinematographic analysis of serial sections.
The work reported has been done primarily on three animals which were sectioned perpendicular to the body axis completely from the snout through the nerve ring. Approximately 1,100 120 nm sections were required to cover this distance in the adult. Shorter series, encompassing only the nerve ring (500 50 nm sections) or only the cephalic end of the animal from the tip to the beginning of the oesophageal musculature (about 200 50 nm sections) were made in five additional cases in order to determine the extent of variability from animal to animal or to resolve difficulties presented by obliquely cut membrane surfaces.
Observations
General description
The basic body plan of C. elegans conforms to the general description of nematode, given in a numher of accounts (Lee, '65; Crofton, '66; Bird, '71), and is that of a spindle-shaped tube with a thin superficial layer of longitudinally oriented muscles surrounding gut, reproductive, and passive supportive systems which fill nearly all of the interior. The central nervous system, located within the relatively short cephalic region, integrates input from the anterior senory organs and provides outpul to the rest of the body by means of peripheral nerve cords which extend the length of the animal and are connected with each other by circumferential connectives. The most important of the cords as judged by the number of nerve fibers contained is the ventral cord.
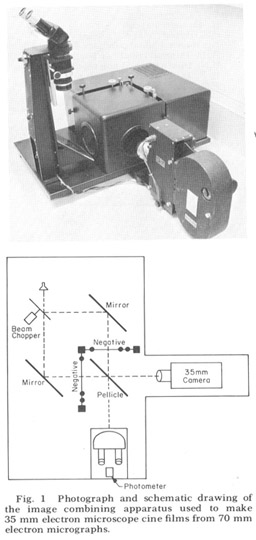 Figure 1. Photograph and schematic drawing of the image combining apparatus used to make 35 mm electron miscroscope cine films from 70 mm electron micrographs.
Figure 1. Photograph and schematic drawing of the image combining apparatus used to make 35 mm electron miscroscope cine films from 70 mm electron micrographs.
| A, Amphid |
LG, Lateral ganglion |
| AC, Amphidial connective |
L.N, Lateral papillary nerve |
| A.C, Amphidial cap process |
LSM, Lateral submedial sense organ |
| AN, Amphidial neuron, nerve |
LSM.C, Lateral submedial cap process |
| AP, Amphidial pouch |
LSM.P, Lateral submedial pocket process |
| A.P, Amphidial pocket process |
M, Muscle fibers, processes |
| Bu, Buccal cavity |
MP, Muscle plate |
| C, Club-shaped dendritic ending, cell of the ventro lateral papillary organ |
MSM, Medial submedial sense organ |
| Cu, Cuticle |
MSM.C, Medial submedial cap process |
| DC, Dorsal nerve cord |
MSM.P, Medial submedial pocket process |
| De, Deirid |
N, Neuron of the nerve ring |
| Des, Desmosome |
NR, Nerve ring |
| DN, Subdorsal papillary nerve |
OE, Oesophagus |
| EC, Excretory canal |
TB, Tubular body termination of the LSM organ |
| ES, Extracellular space |
V, Vesicles |
| F, Finger processes of the amphid |
VG, Ventral ganglion |
| Gl, Glial cytoplasm |
VL, Ventrolateral sense organ |
| H, Hypodermal cell |
VL.C, Ventrolateral cap process |
| IL, Internal labial papillary sense organ |
VL.P, Ventrolateral pocket process |
| IL.C, Internal labial cap process |
VN, Subventral papillary nerve |
| IL.N, Internal labial non-rootleted dendrite |
1, 1-cell process, cell body |
| IL.P, Internal labial pocket process |
2, 2-cell process, cell body |
| IL.R, Internal labial rootleted dendrite |
3, 3-cell process, cell body |
| |
60, 60-cell process, cell body |
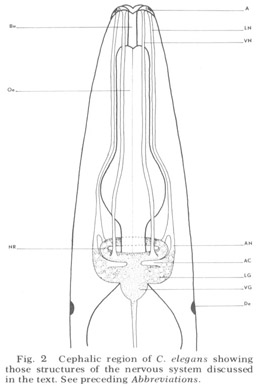 Figure 2. Cephalic region of C. elegans showing those structures of the nervous system discussed in the text. See preceding Abbreviations.
Figure 2. Cephalic region of C. elegans showing those structures of the nervous system discussed in the text. See preceding Abbreviations.
An overall survey of the nervous system of nematodes has been given most recently by Bird ('71). That portion discussed here is diagrammed schematically for C. elegans in figure 2. Basically it consists of the central brain mass of neuropil, called the nerve ring because it completely encircles the axially placed oesophagus, and associated cells and ganglia. The main input to the nerve ring, consistently observed in all species of nematodes and first described by Btschli (1874), comes from six hexagonally placed aggregations of sensory organs on the snout by means of six longitudinal papillary nerves. In C elegans, the papillary nerves do not enter the ring anteriorly, as described in the light microscopic studies of other nematodes, but instead bypass it, then make a full U-turn and enter into the nerve ring neuropil from the rear (fig 28). Other sources of sensory input are (i) the cervical papillae or deirids, two laterally placed presumed pressure-sensitive organs located within the cuticle just posterior to the nerve ring; (ii) phasmids, presumed chemoreceptor organs of the tail which send processes to the nerve ring by means of the ventral cord; (iii) anal papillae. In addition, there are numerous extra receptors in the anal region of males which are presumably used during copulation.
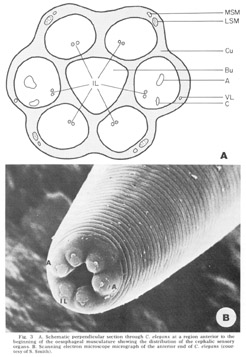 Figure 3. A. Schematic perpendicular sectino through C. elegans at a region anterior to the beginning of the oesophageal musculature showing the distribution of the cephalic sensory organs. B. Scanning electron miscroscope micrograph of the anterior end of C. elegans (courtesy of S. Smith).
Figure 3. A. Schematic perpendicular sectino through C. elegans at a region anterior to the beginning of the oesophageal musculature showing the distribution of the cephalic sensory organs. B. Scanning electron miscroscope micrograph of the anterior end of C. elegans (courtesy of S. Smith).
The anterior sense organs are located near the tip of the animal (fig 3) and are classically divided into two types. Various papillary (or setaceous) organs, assumed to be mechanoreceptors, are located on or near each of the six lips and are innervated by one or two sensory neurons. The amphids, assumed to be chemoreceptors because of their marked openings to the exterior of the animal, are located one on each of the lateral lips. They are larger than the papillary organs and are innervated by many more neurons. All organs are connected to the CNS by the six papillary nerves (fig 2), one to each lip, which contain the anterior processes of their centrally located bipolar neurons.
Detailed structure of the organs in C. elegans
In C. elegans there are 16 papillary organs and two amphids. Each of the submedial lips (two subdorsal and two subventral) has three distinct cuticu!ar papillary organs, one (IL) containing two ciliated dendrites in a clearly protruding papilla at the tip and two (MSM and LSM) slightly posterior, each containing one dendrite. These three organs are joined to the CNS by a common papillary nerve (fig 2). The lateral lips have two cuticular papillary organs each, one (IL) which is structurally homologous to the submedial IL organs, the other (VL) placed more posteriorly and slightly ventrally but not structurally homologous with either the LSM or the MSM organ of the submedial lips. In addition, the lateral lips have a cyathiform amphid which contains 12 ciliated dendritic endings. The amphidial and lateral papillary dendrites travel to the CNS together in a common lateral nerve, which is therefore larger than the submedial nerves. The lateral nerve contains, in addition, a single neuron fiber which enters into the oesophagus near the opening of the dorsal oesophageal gland as described in other nematodes (Chitwood and Chitwood, '50).
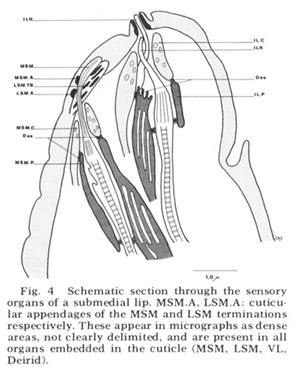 Figure 4. Schematic section through the sensory organs of a submedial lip. MSM.A, LSM.A cuticular appendages of the MSM and LSM terminations respectively. These appear in micrographs as dense areas, not clearly delimited, and are present in all organs embedded in the cuticle (MSM, LSM, VL, Deirid).
Figure 4. Schematic section through the sensory organs of a submedial lip. MSM.A, LSM.A cuticular appendages of the MSM and LSM terminations respectively. These appear in micrographs as dense areas, not clearly delimited, and are present in all organs embedded in the cuticle (MSM, LSM, VL, Deirid).
Each of these sensory organs has two accessory processes surrounding its dendries, a feature first described in Ascaris by Goldschmidt ('03). The relationship of these cells to the ciliated dendrites is shown schematically in figure 4 for two papillary organs in a submedial lip. The cap cell process (Goldschmidt's "Geleitzelle") surrounds the cilium only at its distal end and contains a few small, irregularly shaped electron-lucent vesicles. It forms a continuous desmosomal junction with itself, with the pocket cell process, and with neighboring cells of the lip tissue but not with the cilium. The pocket cell process (Goldschmidt's "Stutzzelle") has a larger terminal swelling with what appear to be extensive lamellar inclusions (the "myelin figures" of Yuen ['67]). In some sections these appear closed, but in others they seem to be continuous with the outer cell membrane and protrude into the extracellular space surrounding the cilium (fig 5B). Posteriorly the pocket cell process forms a continuous desmosomal junction with each of the ciliated dendrites at its point of penetration (fig 11C). Consequently the entire ciliary portion of the dendrite distal to the origin of its microtubules is isolated within an extracellular space formed by the two accessory processes. The cell bodies of most of the cap and pocket processes are located posteriorly in the vicinity of the first oesophageal swelling (fig 2). They are joined to their anterior specializations by fine fibers which travel within the papillary nerves and are morphologically indistinguishable from nerve fibers.
In addition to the classically described papillary and amphidial organs the lips of C. elegans contain a number of other endings of processes which also travel in the papillary nerves. All but one of these are nervous, as judged by their posterior projection into the nerve ring, and several contain ciliary elements suggestive of sensory function. However, unlike the papillary and amphidial dendries they are not surrounded by accessory cells and do not terminate at the cuticle Instead they end internally with a variety of shapes, some of them highly convoluted.
Internal labial papillae. The internal labial papillae are sImilar in all six lips. They consist (fig 5) of the two associated cell processes and, as in all cases in which they have been studied (Yuen, '67; Mc-Laren, '72; DeGrisse et al., 74; 8aldwin and Hlrschmann, '74), two ciliated dendrites of about the same diameter (130 nm), each ending at a slightly different level within the structure. One of these dendrites (ILR) possesses a rootlet four microns in length with a characteristic asymmetrical banding pattern (fig 5D), while the other (ILN) has a rudimentary rootlet observed only in longitudinal sections. The tips of these cilia, which are the most anterior ending of the sensory neurons, are exposed to the exterior through a pore in a cuticular cap which protrudes from the front of the animal at an angle of about 30 from the longitudinal (figs 3B, 5A). No cuticular sheath has been observed surrounding these dendrites. The exact terminal course of the two dendrites, not easily represented schematically (fig 4), consists of a series of reproducible right angle bends just beneath the protrusion. The result is that the lLR fiber ends abutting perpendicular to the ILN fiber at the inner margin of the cuticle and within the pore whereas the ILN fiber proceeds to the end of the channel (fig 5A, inset). At its proximal point of entry into the pocket cell the ILN dendrite possesses a swelling m which there are a number of electron lucent vesicles of about 65 nm diameter. These are the only papillary organs which possess a marked extracellular space into which fine filaments from the pocket cell processes project (fig 5B). This space, though distinct, is small compared to that found surrounding dendrites in insect pressure-sensitive sensilia (e.g., Gnatzy and Schmidt, '71) and occurs from the distal rootlet region of the ILR dendrite to the cuticular opening on the snout.
Lateral submedial papillae. These organs (fig 6) have no cuticular protrusion. The sensory organ consists of a single nonrootleted ciliated fiber (LSM) embedded terminally in the cuticle for a distance of two and one-half microns, plus the two associated cell processes. Compared to the internal labial organ, the lateral submedial has only a very narrow extracellular space between its dendrite and the laminated associated cell. At its terminal end the distal segment swells to a diameter of about 350 nm and contains 10 to 15 diffuse electron dense rodlike structures which are not always discrete but sometimes merge into one another eiher by contact or by interposition of additional dense material. Each of the rods is surrounded by a complement of about five microtubules (figs 6B, 7B). The general appearance of this terminal specialization is similar to the tubular body structure frequently observed in insect mechanoreceptors. As in the amphidial and ILN dendrites, there is a swelling filled with 65 nm diameter vesicles at the entry of this fiber into the proximal part of its pocket cell.
It is interesting in several respects to compare this organ in C. elegans with the homologous organ of Ascaris described by Goldschmidt ('03). First, Goldschmidt found the nerve fiber supplying the organ to be thick relative to the other nerve fibers in the submedial bundles. This is likewise true in C. elegans. Second, the pocket cell of this organ in Ascaris was found not to end in a monopolar cell body between the nerve ring and snout as is the case with other papillary associated cells, but rather to have its cell body at the nerve ring, where it takes on the nature of a glial cell, ensheathing the neuropil of the ring with a thin layer of its cytoplasm and separating it from the surrounding mesodermal tissue. This is also true for the lateral submedial pocket cells in C. elegans (figs 17-19, 24, 28, 29, 33). ThIrd, in his original paper ('03) Goldschmidt was unable to locate cell bodies for the nerve fibers of these organs anterior to the nerve ring. In a later paper (Goldschmidt, '08) he amended this finding and called them his cells 50 in the subventral papillary ganglia, with no explicit mention of them sub- dorsally In C. elegans we have found that the nerve cell bodies of these organs are located just posterior to the nerve ring subdorsally and just anterior to it subventrally (fig 18, LSM). These are the only cell bodies of homologous sensory neurons which do not occupy corresponding positions about the nerve ring in C. elegans.
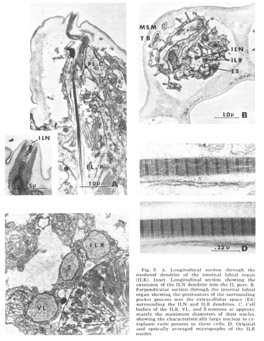 Figure 5. A. Longitudinal section through the rootleted dendrite of the internal labial organ (ILR). Inset: Longitudinal section showing the extension of the ILN dendrite into the IL pore. B. Perpendicular sectino through the internal labial organ showing the protrusions of the surrounding pocket process into the extracellular space (ES) surrounding the ILN and ILR dendrites. C. Cell bodies of the ILR, VL, and 2-neurons at approximately the maximum diameters of their nuclei, showing the characteristically large nuclear to cytoplasm ratio present in these cells. D. Original and optically averaged micrographs of the ILR rootlet.
Figure 5. A. Longitudinal section through the rootleted dendrite of the internal labial organ (ILR). Inset: Longitudinal section showing the extension of the ILN dendrite into the IL pore. B. Perpendicular sectino through the internal labial organ showing the protrusions of the surrounding pocket process into the extracellular space (ES) surrounding the ILN and ILR dendrites. C. Cell bodies of the ILR, VL, and 2-neurons at approximately the maximum diameters of their nuclei, showing the characteristically large nuclear to cytoplasm ratio present in these cells. D. Original and optically averaged micrographs of the ILR rootlet.
Medial submedial papillae Like the lateral submedial organs, these also have no cuticular protrusion from the front of the animal In contrast, they do possess a long striated rootlet with the same ultrastructural banding pattern as observed in the rootleted internal labial dendrite (fig 7). The single ciliated fiber (MSM) ends in an elongated 130 nm diameter termination which is embedded in the cuticle for a distance of about two microns. The terminal specialization of the dendrite is difficult to classify in terms of known invertebrate receptors. That portion embedded in the cuticle has a smooth surface toward the outside of the lip, whereas on the inside it is highly crenated. Within the termination there are long discrete electron dense rods. Four of these extend throughout the embedded ending and are located at the corners of a square. Four others extend for only the proximal half of the ending and are located at the corners of a slightly larger square rotated 45 with respect to the first (figs 6B, 7B). No microtubules have been observed in perpendicular sections of that part of the dendrite embeded in the cuticle.
Ventrolateral papillae As in Ascaris, the ventrolateral organ (fig 8) is distinct from either the lateral or medial submedial. The dendrite (VL) is surrounded by cap and pocket cell processes, but its terminal cuticle-embedded portion is much shorter than in either submedial organ and is more nearly spherical (about 300 nm in diameter). A further difference is that there is but a single very electron dense inclusion with about ten microtubules positioned only about its periphery (fig 8B). A similar type of terminal specialization, with a larger number of surrounding microtubules, is observed in the classically described deirids (fig 8C), two cervical sense organs conventionally assumed to be pressure receptors and located laterally within the cuticle, one on each side of the animal, just posterior to the nerve ring.
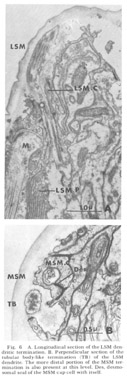 Figure 6. A. Longitudinal section of the LSM dendritic termination. B. Perpendicular section of the tubular body-like termination (TB) of the LSM dendrite. The more distal portion of the MSM termination is also present at this level. Des, desmosomal seal of the MSM cap cell with itself.
Figure 6. A. Longitudinal section of the LSM dendritic termination. B. Perpendicular section of the tubular body-like termination (TB) of the LSM dendrite. The more distal portion of the MSM termination is also present at this level. Des, desmosomal seal of the MSM cap cell with itself.
The ventrolateral organ is of particular comparative interest because in Ascaris it was shown by Goldschmidt to consist of two neurons, one as described, surrounded by the usual cap and pocket cell processes, and one just outside of these associated fibers and ending bluntly against, not within, the cuticle. Goldschmidt also stated that in one special case he observed that this latter dendrite appeared swollen into a club-shape beneath the cuticle. We observe just such a club-shaped ending within the lateral lip tissue in a slightly ventrolateral position (figs 3, 9). This dendrite has a normal striated rootlet and cilium. It is unique in that its terminal specialization involves no inclusions, but simply a flaccid swelling. The distal end of this swelling breaks up into a number of finger-like projections arranged in a petal-like formation which indent the cap cell of the lateral internal labial organ of the same lip (figs 8B, 15C,D). A further peculiarity of this neuron is that its cell body is quite distinct from those of the other papillary neurons, both in size (fig 9B) and in its projection into the nerve ring. Structurally, both the nucleus and cell body are large compared with those of the other papillary nucleus, although the cytoplasm is similar in appearance. In projecting into the nerve ring, the nerve fiber does not enter with the others in the characteristic papillary nerve U-turn mentioned above. Whether this dendrite represents a regression in C. elegans of the outer ventrolateral dendrite of a more basic organ, exemplified in Ascaris, or is just another of the dendrites which are not differentiated classically and which end in the lip tissue is an open question.
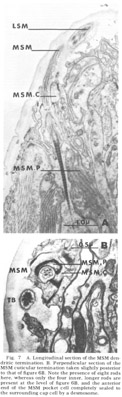 Figure 7. A. Longitudinal section of the MSM dendritic termination. B. Perpendicular section of the MSM cuticular termination taken slightly posterior to that of figure 6B. Note the presence of eight rods are present at the level of figure 6B, and the anterior end of the MSM pocket cell completely sealed to the surrounding cap cell by a desmosome.
Figure 7. A. Longitudinal section of the MSM dendritic termination. B. Perpendicular section of the MSM cuticular termination taken slightly posterior to that of figure 6B. Note the presence of eight rods are present at the level of figure 6B, and the anterior end of the MSM pocket cell completely sealed to the surrounding cap cell by a desmosome.
Amphids The two amphids, one on each laleral lip, are larger and more complicated than the papillary organs. Like them, they are composed of a cap and a pocket cell process, although the extracellular space they surround (the amphidial pouch) is larger and in more dIrect contact with the exterior (fig 10). Instead of one or two neuronal dendrites, the amphidial pouch contains eleven. A twelfth ciliated dendrite invades the pockel cell without ever entering the pouch itself. These dendrites are shown in figure 12. Like the papillary dendrites they enter the pouch by proximal penetration of the pocket cell, where they are sealed with a continuous desmosomal junction (fig. 11C). The pouch itself contains an amorphous, slightly electron dense material which surrounds the 11 dendrites contained within it. Vesicles of uniform diameter (about 65 nm) but varying degrees of electron opacity are commonly seen in many of the dendrites just proximal to their entry into the extracellular space and near to the desmosomal junction (figs 10, 11C). Most often these vesicles are tightly clustered either at the membrane or internally. All dendrites are approximately 350 nm in diameter and possess a rudimentary rootlet, seen only in longitudinal sections (fig 10). They possess microtubules in a configuration similar to that described by Hansen and Heumann ('71) in Phormia, basically a 9 x 2 + m arrangement, where m varies from 1 to 6, with the extra tubules interior to the circle of doublets.
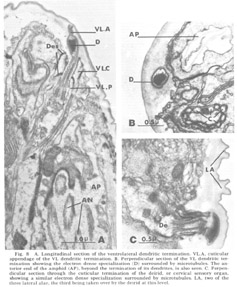 Figure 8. A. Longitudinal section of the ventrolateral dendritic termination. VL.A, cuticular appendage of the VL dendritic termination. B. Perpendicular section of the VL dendritic termination showing the electron dense specialization (D) surrounded by microtubules. The anterior end of the amphid (AP), beyond the termination of its dendrites, is also seen. C. Perpendicular section through the cuticular termination of the deirid, or cervical sensory organ, showing a similar electron dense specialization surrounded by microtubules. LA, two of the three lateral alae, the third being taken over by the deirid at this level.
Figure 8. A. Longitudinal section of the ventrolateral dendritic termination. VL.A, cuticular appendage of the VL dendritic termination. B. Perpendicular section of the VL dendritic termination showing the electron dense specialization (D) surrounded by microtubules. The anterior end of the amphid (AP), beyond the termination of its dendrites, is also seen. C. Perpendicular section through the cuticular termination of the deirid, or cervical sensory organ, showing a similar electron dense specialization surrounded by microtubules. LA, two of the three lateral alae, the third being taken over by the deirid at this level.
One of the most striking features of the amphid, observable only in complete sets of serial sections, is the constancy of its structure from animal to animal. There are three types of dendrite, the same type occurring at a corresponding location in all animals studied, so that a single transverse section which contains all dendrites is sufficient to identify each of them unambiguously.
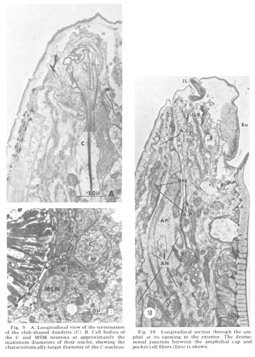 Figure 9. A. Longitudinal view of the termination of the club-shaped dendrite (C). B. Cell bodies of the C and MSM neurons at approximately the maximum diameters of their nuclei, showing the characteristically larger diameter of the C-nucleus.
Figure 9. A. Longitudinal view of the termination of the club-shaped dendrite (C). B. Cell bodies of the C and MSM neurons at approximately the maximum diameters of their nuclei, showing the characteristically larger diameter of the C-nucleus.
Figure 10. Longitudinal section through the amphid at its opening to the exterior. The desmosomal junction between the amphidial cap and pocket cell fibers (Des) is shown.
The three dendrites of the first category (1-3 in fig 12) give rise to terminal portions which do not end after the termination of the microtubules, but instead leave the pouch and reinvade the pocket cell. They continue anteriorly as long thin filamentous extensions but still within the pocket cell. Slightly anterior to figure 11A these extensions reach halfway around the snout dorsally and ventrally, approaching their counterparts from the other lateral lip at the medial poles of the animal. Of these dendrites, 1 and 2 form a single filamentous extension each, while fiber 3 branches posterior to its microtubules to form a pair. The eight dendrites of the second category (4-11 in fig 12) end within the pouch at different but characteristic levels shortly after the termination of their microtubules. Two of these (4 and 5), like fiber 3, branch to form two cilia each, whereas the rest are singlets. The one dendrite of the third category (12 in fig 12) contains microtubuiar inclusions like those of the other fibers, but instead of entering the pouch it remains within the pocket cell, sending out on the order of 50 finger-like projections which indent the pocket cell membrane (figs 11A, B, 12). These projections probably correspond to the "microvilli" observed by Baldwin and Hirschmann ('73) in the amphid of M. incognita.<br><br>
Other anterior endings. In addition to the club-shaped ending described above as part of the ventrolateral organ, the lips of C. elegans contain four additional types of non-cuticular ending from processes contained within the papillary nerves. Three of these, whose cell bodies are labeled 1, 2, and 3 in figure 18, are apparently nervous since they project through the papillary U-turn into the nerve ring. Cells of type 1, in the subdorsal and subventral papillary nerves only, become associated with the MSM papillary organs in a peculiar manner, having a sheet-like ending along the medial surface of the pocket cells of these organs (fig 15A). Cells of type 2 end without specialization next to the oesophagus near the anterior end of its musculature. Cells of type 3, present only subdorsally, possess a ciliary ending with a rudimentary rootlet. Although the 3 fibers travel in the subdorsal bundles for most of the length of the cephalic region, they undergo a right angle turn within the lips, so that their rootlet lies nearly perpendicular to the body axis, and proceed to their neighboring lateral lips. Once there they turn anteriorly again and branch to form a number of long, fine processes which deeply invaginate the cap cell of the lateral internal labial sense organ (figs 15B-D).
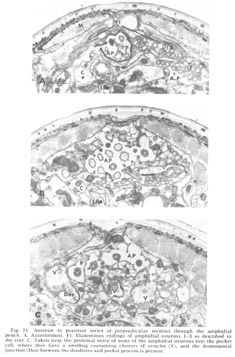 Figure 11. Anterior to posterior series of perpendicular sections through the amphidial pouch. A. Anteriormost. Fi: filamentous endings of amphidial neurons 1-3 as described in the text. C. Taken near the proximal entry of some of the amphidial neurons into the pocket cell, where they have a swelling containing clusters of vesicles (V), and the desmosomal junction (Des) between the dendrites and pocket process is present.
Figure 11. Anterior to posterior series of perpendicular sections through the amphidial pouch. A. Anteriormost. Fi: filamentous endings of amphidial neurons 1-3 as described in the text. C. Taken near the proximal entry of some of the amphidial neurons into the pocket cell, where they have a swelling containing clusters of vesicles (V), and the desmosomal junction (Des) between the dendrites and pocket process is present.
 Figure 12. Schematic section of the amphidial pouch at a level corresponding to figure 11B. The relative positions of the dendrites are constant from animal to animal, enabling each of them to be identified unambiguously in a single section.
Figure 12. Schematic section of the amphidial pouch at a level corresponding to figure 11B. The relative positions of the dendrites are constant from animal to animal, enabling each of them to be identified unambiguously in a single section.
Figure 13. Cell bodies of the amphidial pocket cell (A.P) and some amphidial neurons (AN). Comparison with figures 5C and 9B shows the characteristically richer cytoplasm of the amphidial neurons compared with the papillary neurons, their larger nuclear and cell body radii and their smaller nuclear to cytoplasm ratio.
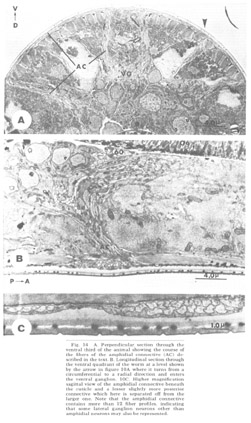 Figure 14. A. Perpendicular section through the ventral third of the animal showing the course of the fibers of the amphidial connective (AC) described in the text. B. Longitudinal section through the ventral quadrant of the worm at a level shown by the arrow in figure 10A where it turns from a circumferential to a radial direction and enters the ventral ganglion. 10C. Higher magnification sagittal view of the amphidial connective beneath the cuticle and a lesser slightly more posterior connective which here is separated off from the larger one. Note that the amphidial connective contains more than 12 fiber profiles, indicating that some lateral ganglion neurons other than amphidial neurons may also be represented.
Figure 14. A. Perpendicular section through the ventral third of the animal showing the course of the fibers of the amphidial connective (AC) described in the text. B. Longitudinal section through the ventral quadrant of the worm at a level shown by the arrow in figure 10A where it turns from a circumferential to a radial direction and enters the ventral ganglion. 10C. Higher magnification sagittal view of the amphidial connective beneath the cuticle and a lesser slightly more posterior connective which here is separated off from the larger one. Note that the amphidial connective contains more than 12 fiber profiles, indicating that some lateral ganglion neurons other than amphidial neurons may also be represented.
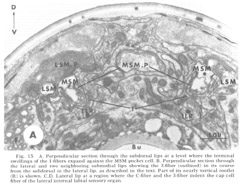
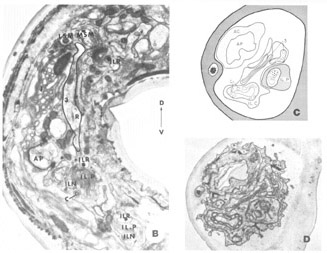 Figure 15. A. Perpendicular section through the subdorsal lips at a level where the terminal swellings of the 1-fibers expand against the MSM pocket cell. B. Perpendicular section through the lateral and two neighboring submedial lips showing the 3-fiber (outlined) in its course from the subdorsal to the lateral lip, as described in the text. Part of its nearly vertical rootlet (R) is shown. C, D. Lateral lip at a region where the C-fiber and the 3-fiber indent the cap cell fiber of the lateral internal labial sensory organ.
Figure 15. A. Perpendicular section through the subdorsal lips at a level where the terminal swellings of the 1-fibers expand against the MSM pocket cell. B. Perpendicular section through the lateral and two neighboring submedial lips showing the 3-fiber (outlined) in its course from the subdorsal to the lateral lip, as described in the text. Part of its nearly vertical rootlet (R) is shown. C, D. Lateral lip at a region where the C-fiber and the 3-fiber indent the cap cell fiber of the lateral internal labial sensory organ.
A fourth cell type, not described in Ascaris but which has been found to contribute a fiber to the papillary nerves of C. elegansis labeled as Cell 60 in figure 18. The fiber of this monopolar cell separates off from the rest of the papillary bundle just anterior to the nerve ring and does not enter the U-turn. Posterior to its separation it swells into abroad sheet like expansion which is immediately apposed to the oesophagus and which extends for about 60 circumferentially under nearly all of the nerve ring neuropil (figs 26, 33). The sheets of some of these cells are observed to make what appear to be very broad gap junction contacts with at least one fiber of the nerve ring whose cell body is designated as DM in figure 18. The 60-cell bodies are different from all others observed, being densely packed with small dark granules and non-membrane bound clear areas (fig 16). Anteriorly, the 60-processes end without specialization next to the oesophagus near the anterior end of its musculature.
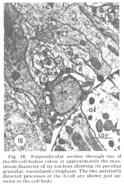 Figure 16. Perpendicular section through one of the 60-cell bodies taken at approximately the maximum diameter of its nucleus showing its peculiar granular, vacuolated cytoplasm. The two anteriorly directed processes of the 3-cell are shown just anterior to the cell body.
Figure 16. Perpendicular section through one of the 60-cell bodies taken at approximately the maximum diameter of its nucleus showing its peculiar granular, vacuolated cytoplasm. The two anteriorly directed processes of the 3-cell are shown just anterior to the cell body.
Cell body locations
The neuron cell bodies of one of four completely sectioned nerve rings are shown in three oblique schematic views of the same animal in figure 17. This figure encompasses the region of the nerve ring from the anterior-most neurons through the beginning of the smaller ventral ganglion, some cells of which are present in the posterior regions of the figure. All of those cells which have anteriorly directed processes are identified by the nerve bundles of which they are a part. Figure 18 shows the cell body locations in the nerve ring and anterior part of the ventral ganglion of two animals in a diagram representing the worms cut along their ventral midlines and then flattened out into a plane. In each case, identification of a cell body was made by following its peripheral process to its terminal specialization on the lips in complete series of electron micrographs. In the figure, neurons of the papillary bundles are identified both by the bundle of which they are a part and their sensory nature. One of the noteworthy features is the high degree of bilateral symmetry in cell body position. Comparison of different animals has shown that these cells are distributed in a similar manner in all cases studied.
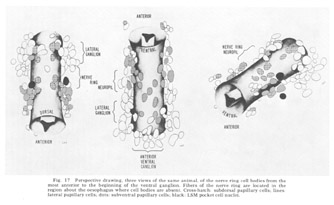 Figure 17. Perspective drawing, three views of the same animal, of the nerve ring cell bodies from the most anterior to the beginning of the ventral ganglion. Fibers of the nerve ring are located in the region about the oesophagus where cell bodies are absent. Cross-hatch: subdorsal papillary cells; lines: lateral papillary cells; dots: subventral papillary cells; black: LSM pocket cell nuclei.
Figure 17. Perspective drawing, three views of the same animal, of the nerve ring cell bodies from the most anterior to the beginning of the ventral ganglion. Fibers of the nerve ring are located in the region about the oesophagus where cell bodies are absent. Cross-hatch: subdorsal papillary cells; lines: lateral papillary cells; dots: subventral papillary cells; black: LSM pocket cell nuclei.
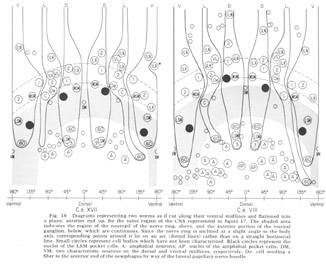 Figure 18. Diagrams representing two worms as if cut along their ventral midlines and flattened into a plane, anterior end up, for the same region of the CNS represented in figure 17. The shaded area indicates the region of the neuropil of the nerve ring, above, and the anterior portion of the ventral ganglion, below, which are continuous. Since the nerve ring is inclined at a slight angle to the body axis, corresponding points around it lie on an arc (dotted lines) rather than on a straight horizontal line. Small circles represent cell bodies which have not been characterized. Black circles represent nuclei of the LSM pocket cell. A: amphidial neurons; AP: nuclei of the amphidial pocket cell; VM: two characteristic neurons on the dorsal and ventral midlines, respectively; Oe: cell sending a fiber to the anterior end of the oesophagus by way of the lateral papillary nerve bundle.
Figure 18. Diagrams representing two worms as if cut along their ventral midlines and flattened into a plane, anterior end up, for the same region of the CNS represented in figure 17. The shaded area indicates the region of the neuropil of the nerve ring, above, and the anterior portion of the ventral ganglion, below, which are continuous. Since the nerve ring is inclined at a slight angle to the body axis, corresponding points around it lie on an arc (dotted lines) rather than on a straight horizontal line. Small circles represent cell bodies which have not been characterized. Black circles represent nuclei of the LSM pocket cell. A: amphidial neurons; AP: nuclei of the amphidial pocket cell; VM: two characteristic neurons on the dorsal and ventral midlines, respectively; Oe: cell sending a fiber to the anterior end of the oesophagus by way of the lateral papillary nerve bundle.
In marked contrast to the observations on Ascaris (Goldschmidt '08), we find only a very limited aggregation of cell bodies into the classical ganglia in C. elegans. Rather, they seem to form a loosely packed continuum of cell bodies which are occasionally separated from the surrounding hypodermal cells by thin glial processes of the LSM pocket cells, but more often are in direct contact with them.The ventral and lateral ganglia are differentiated by dividing the animal into four imaginary quadrants, two medial and two lateral, delineating their boundaries by the four symmetrical cephalic muscle bundle entries (see discussion of cephalic musculature). The lateral ganglia are taken to be composed of those cell bodies, including the amphidial cells, in the lateral quadrants, and the ventral ganglion of those cell bodies located in the ventral quadrant and clustered about the ventral posterior extension of the nerve ring neuropil. The absence of the commonly called papillary ganglia is easily observed in figure 18, where the neurons contributing to the papillary nerves are seen to be present both anterior and posterior to the nerve ring neuropil.
The correspondence in position around the ring of cells having homologous endings in the snout is apparent, with the exception, as mentioned, of the LSM nerve cells, which have their cell bodies anterior to the ring subventrally and posterior subdorsally. Of particular interest is that the nerve cell bodies of the VL organs are nowhere near the corresponding position of either of the two submedial organ neurons with which they might be homologous on the basis of classical studies, namely, the LSM and MSM. The cell bodies of the rootleted club-shaped dendrites (C) are located in a position laterally corresponding to the MSM neurons, but as has been described they are so distinct from the other papillary neurons, both in structure nad in nerve ring projection that they can not be described as being homologous with them.
The subdorsal LSM and 3-neurons and the 60-cells are unusual in structure. First, whereas all other cells contributing to the papillary bundle are anterior to the ring, these are posterior. Secondly, all other neurons are bipolar with one anteriorly and one posteriorly directed process. In contrast, the subdorsal LSM neurons are monopolar with a branch point at the U-turn, one branch traveling to the corresponding lip, the other entering the ring just posterior to the U-turn. The 60-cells are monopolar. The subdorsal 3- neurons are bipolar, but with both processes directed anteriorly, one going to the snout, the other to the nerve ring just posterior to the subdorsal U-turn. This difference has been found consistently with but a single exception. On one side of one animal, the 3-cells was found to be monopolar with one branch point, the two fibers coming from it duplicating the courses of the two normally unbranched fibers.
Cephalic Musculature
Only much more general information is available concerning the pattern of nematode muscle innervation, which has been known from the work of Schneider (1860) to be peculiar in that the muscles send processes to the CNS rather than vice versa. The structure of the muscle cell itself has been investigated for over a century (reviewed by Debell, 65) and has been shown to consist of three parts an outer contractile portion apposed longitudinally just inside the cuticle; an intermediate "belly," containing the nucleus, other cellular organelles, and large supplies of glycogen; and an inner fiber portion which projects into the CNS (nerve ring or cords, depending on its location). The organization of the myofilaments in the contractile portion of the cell was investigated by Rosenbluth ('65a; 67) who found that they are arranged so as to produce an obliquely striated muscle as has been observed in a number of invertebrates. In this type of muscle the rectangular sarcomere typical of vertebrate striated muscle is in effect sheared along a plane parallel to the filaments, with the continuous Z-band plate being replaced by discrete posts which serve as points of attachment for the thin filaments.
As is generally true with the smaller terrestrial nematodes, somatic musculature in C. elegans is of the meromyarian-platymyarian type (Bird, '71), in which there is a single layer of sarcomeres beneath the cuticle (fig 19). When cut perpendicular to the body axis (fig 20A) the filaments of a muscle cell are seen to be grouped into from one to five sarcomeres. Each extends nearly the length of the cell and is separated from its neighbors by the Z-plane, into which the electron dense attachment posts are inserted at approximately one micron intervals along the animal. A higher magnification perpendicular view of a single sarcomere (fig 20B) reveals the expected banding pattern, a narrow central region in which only thick filaments are present (H band), two surrounding broader regions (A-bands) in which both thick and thin are present, and outside of them the two narrow regions consisting only of thin filaments (I bands). A longitudinal section through several sarcomeres of this muscle (fig 20C) illustrates the shear required to produce the oblique striated pattern from the striated one.
Several observations indicate thai in C. elegans body and cephalic somatic muscles may comprise two developmentally different muscle systems (i) body muscles send their innervation processes only to the nerve cords, whereas the processes from the cephalic muscle cells within each quadrant form a bundle of fibers which enter the nerve ring and there form chemical synapses directly with primary sensory cells as well as with unidentified interneurons; (ii) we possess a number of independently isolated, presumably point mutants in which body musculature is apparently normal but in which there is a defect in some or all of the cephalic musculature in the adult animal leading to partial or complete paralysis of the cephalic region. In such animals the volume normally occupied by the muscle cells is partially taken over by the very characteristically appearing hypodermal cells; (iii) we have isolated one example of a complementary mutant in which the cephalic region is fully active but in which the body muscles fail to function in the adult.
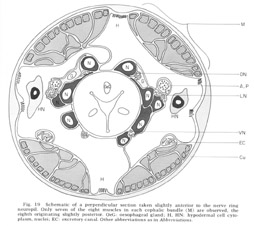 Figure 19. Schematic of a perpendicular section taken slightly anterior to the nerve ring neuropil. Only seven of the eight muscles in each cephalic bundle (M) are observed, the eigth originating slightly posterior. OeG: oesophageal gland; H, HN: hypodermal cell cytoplasm, nuclei; EC: excretory canal. Other abbreviations as in Abbreviations.
Figure 19. Schematic of a perpendicular section taken slightly anterior to the nerve ring neuropil. Only seven of the eight muscles in each cephalic bundle (M) are observed, the eigth originating slightly posterior. OeG: oesophageal gland; H, HN: hypodermal cell cytoplasm, nuclei; EC: excretory canal. Other abbreviations as in Abbreviations.
The cephalic region of C. elegans contains 32 muscle cells arranged into four submedial groups of eight (figs 19, 21). The positioning of the cells, nuclei, contractile portions, and innervation processes within any group represents a mirror reflection of the neighboring group, consistent with the basically bilateral symmetry of the animal observed both in the sensory organs and in their innervations of the nerve ring. The cells of each group of eight can be divided according to the locations of their contractile portions into an anterior four and a posterior four, or alternatively into a more medial four and a more lateral four. Although there is overlap, these divisions are always unambiguous, and they have a significance which is revealed in the way the muscles receive their innervation in the nerve ring (figs 22-27). Each muscle cell sends a process radially toward the oesophagus just behind the neuropil of the nerve ring. This process spreads out into a very thin sheet which extends circumferentially and longitudinally forward and is inserted between the 60-fiber sheets and nerve ring. Synapses from ring nerve fibers occur on small projections which are sent up toward the ring from the edge of this sheet in one case at the point of entry (fig 33), but mainly in the terminal medial and lateral regions (figs 26,27, 29, 32D).
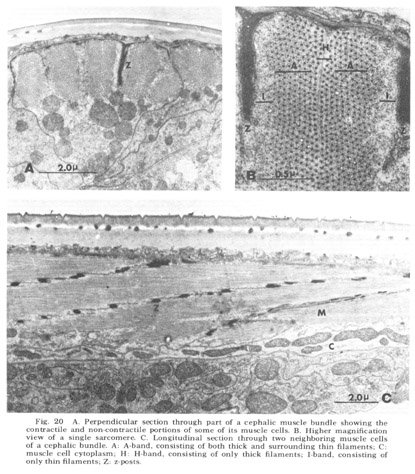 Figure 20. A. Perpendicular section through part of a cephalic muscle bundle showing the contractile and non-contractile portions of some of its muscle cells. B. Higher magnification view of a single sarcomere. C. Longitudinal section through two neighboring muscle cells of a cephalic bundle. A: A-band, consisting of both thick and surrounding thin filaments; C: muscle cell cytoplasm; H: H-band, consisting of only thick filaments; I-band, consisting of only thin filaments; Z: z-posts.
Figure 20. A. Perpendicular section through part of a cephalic muscle bundle showing the contractile and non-contractile portions of some of its muscle cells. B. Higher magnification view of a single sarcomere. C. Longitudinal section through two neighboring muscle cells of a cephalic bundle. A: A-band, consisting of both thick and surrounding thin filaments; C: muscle cell cytoplasm; H: H-band, consisting of only thick filaments; I-band, consisting of only thin filaments; Z: z-posts.
The entry pattern of each fiber of the group is symmetric in all quadrants and identical in all animals studied. The four more medial cells expand their sheets medially around the oesophagus, the four more lateral cells laterally (figs, 22, 23). The division of the group of eight cells into four anterior and four posterior is also significant. The sheets themselves are stacked in order so that the more anterior muscle of the four has its sheet nearest the oesophagus, the more posterior just under the nerve ring. The four anterior muscles of the bundle have large swellings immediately before the formation of their sheets behind the nerve ring (figs, 22, 23). These swellings contain no mitochondria or other organelles, but are densely packed with glycogen granules (Rosenbluth '65b). The four more posterior cells on the other hand possess exceedingly slender processes (350 nm) before the formation of theIr sheets, which by electron microscopy seem to contain only small if any amounts of glycogen. These cells have a second pro- jection into the CNS, more posterior into their respective medial cords (fig 25). At these locations the entering processes are much broader and do possess a dense homogeneous glycogen content.
 Figure 21. Diagram of the distribution of the contractile portions of the cephalic muscle cells representing a worm as if cut along its ventral midline and flattened into a plane, posterior end up. Positions of nuclei are indicated by circles. The column of labels to the left represents our identification of muscle cells within a bundle. M, broken lines: medial muscles; L, solid lines: lateral muscles; A: anterior; P: posterior.
Figure 21. Diagram of the distribution of the contractile portions of the cephalic muscle cells representing a worm as if cut along its ventral midline and flattened into a plane, posterior end up. Positions of nuclei are indicated by circles. The column of labels to the left represents our identification of muscle cells within a bundle. M, broken lines: medial muscles; L, solid lines: lateral muscles; A: anterior; P: posterior.
At the medial and lateral poles where the sheets from two of the neighboring groups of eight meet there is formed a very characteristic synaptic region termed the muscle plate (figs. 26, 27). It is largely in this region that the muscle fibers send projections up toward the neuropil, and depending on the muscle cell, branch characteristically to receive theIr synaptic contacts. As has been described by Rosenbluth ('65b) for the innervation of the body somatic musculature by the ventral cord in Ascaris, the synapses do not have the appearance of vertebrate myoneuronal junctions but appear more like highly localized neuroneuronal synapses. Those homologous muscles which we have compared in different animals have shown the same course and branching pattern. Synapses onto the muscles come mainly from the motoneurons. Different muscle cells synapse with different numbers of motoneurons. Although none of the motoneurons has yet been traced to its cell body, the configuration of the neuropil is sufficiently reproducible from animal to animal to permit the conclusion that corresponding muscle cells in different animals are postsynaptic to the same set of fibers. Within the plates homologous muscle cells from the two neighboring quadrants have also been seen to contact one another by means of what appear to be gap junctions (fig 26, inset). We have also identified one case in which a primary sensory cell, the rootleted ciliary cell of the internal labial papilla, synapses dIrectly onto a muscle cell (fig 33, ILR synapsing onto muscle MA1), thus forming an evolutionarily primitive sensory motor synapse.
Neuropil
Consistent with the low phylogenetic status of nematodes, the neuropil of the nerve ring of C. elegans is exceedingly simple. It possesses none of the characteristics of fiber organization commonly associated with central ganglia of higher invertebrates such as distinctive glomeruli, stratifications, or giant fibers (Bullock and Horridge, '65). No doubt this is a reflection of the small number of neurons, each of which except for symmetry may be unique in function. It has been stated (Hyman, '51) that the nerve ring is more like a commisure connecting the lateral ganglia, which themselves correspond to the cerebral ganglia of other Protostomia. This seems not to be the case in C. elegans. Although the nerve ring does, in overall structure, have a commisural appearance in that its fibers travel largely in a circumferential direction, it is also the location of numerous synaptic interactions and the terminations of some identified sensory neurons. Since the lateral ganglia of C. elegans have no neuropil, it is apparent that from a functional point of view the nerve ring itself is the cerebral ganglion.
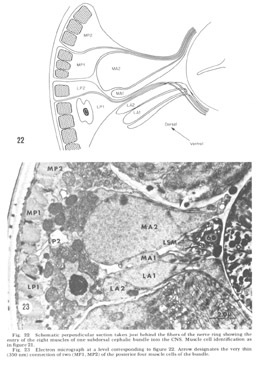 Figure 22. Schematic perpendicular section taken just behind the fibers of the nerve ring showing the entry of the eight muscles of one subdorsal cephalic bundle into the CNS. Muscle cell identification as in figure 21.
Figure 22. Schematic perpendicular section taken just behind the fibers of the nerve ring showing the entry of the eight muscles of one subdorsal cephalic bundle into the CNS. Muscle cell identification as in figure 21.
Figure 23. Electron micrograph at a level corresponding to figure 22. Arrow designates the very thin (350 nm) connection of two (MP1, MP2) of the posterior four muscle cells of the bundle.
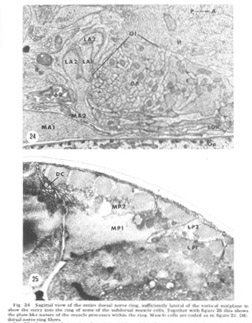 Figure 24. Sagittal view of the entire dorsal nerve ring, sufficiently lateral of the vertical midplane to show the entry into the ring of some of the subdorsal muscle cells. Together with figure 26 this shows the plate-like nature of the muscle processes within the ring. Muscle cells are coded as in figure 21. DR: dorsal nerve ring fibers.
Figure 24. Sagittal view of the entire dorsal nerve ring, sufficiently lateral of the vertical midplane to show the entry into the ring of some of the subdorsal muscle cells. Together with figure 26 this shows the plate-like nature of the muscle processes within the ring. Muscle cells are coded as in figure 21. DR: dorsal nerve ring fibers.
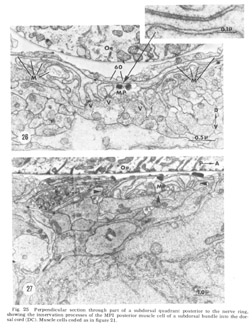 Figure 25. Perpendicular section through part of a subdorsal quadrant posterior to the nerve ring, showing the innervation processes of the MP1 psoterior muscle cell of a subdorsal bundle into the dorsal cord (DC). Muscle cells coded as in figure 21.
Figure 25. Perpendicular section through part of a subdorsal quadrant posterior to the nerve ring, showing the innervation processes of the MP1 psoterior muscle cell of a subdorsal bundle into the dorsal cord (DC). Muscle cells coded as in figure 21.
 Figure 26. Ventral muscle plate, tangential section. Clustering of vesicles in the two symmetrically lacated presynaptic fibers on the midline can be seen, as well as a number of synapses onto muscle sheet processes. Some of the presynaptic darkenings show the more characteristic point-synapse structure on neighboring sections, and all, especially those onto the smallest sheet processes, are highly lacalized, spanning a distance no greater than that occupied by the postsynaptic muscle process. At the center of the plate the muscle processes become so convoluted and branched that they can only be identified in a long series of micrographs. M, the four entering muscle sheets from the medial muscles of the two subventral bundles in order from the oesophagus outward: MA1, MA2, MP1, MP2; 60: the subventral 60-fibers slightly anterior to their cell bodies. Beginnings of the granular, vacuolated cytoplasm characteristic of these cells can be seen. Inset: higher magnification of a different section showing the gap junction formed between the two homologous MA1 cells of the two subventral muscle groups (long arrow).
Figure 26. Ventral muscle plate, tangential section. Clustering of vesicles in the two symmetrically lacated presynaptic fibers on the midline can be seen, as well as a number of synapses onto muscle sheet processes. Some of the presynaptic darkenings show the more characteristic point-synapse structure on neighboring sections, and all, especially those onto the smallest sheet processes, are highly lacalized, spanning a distance no greater than that occupied by the postsynaptic muscle process. At the center of the plate the muscle processes become so convoluted and branched that they can only be identified in a long series of micrographs. M, the four entering muscle sheets from the medial muscles of the two subventral bundles in order from the oesophagus outward: MA1, MA2, MP1, MP2; 60: the subventral 60-fibers slightly anterior to their cell bodies. Beginnings of the granular, vacuolated cytoplasm characteristic of these cells can be seen. Inset: higher magnification of a different section showing the gap junction formed between the two homologous MA1 cells of the two subventral muscle groups (long arrow).
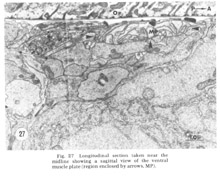 Figure 27. Longitudinal section taken near the midline showing a sagittal view of the ventral muscle plate (region enclosed by arrows, MP).
Figure 27. Longitudinal section taken near the midline showing a sagittal view of the ventral muscle plate (region enclosed by arrows, MP).
The most obvious deviation from a purely commisural nature of the ring occurs in its participation in the integration of sensory input and cephalic muscle motor output. Primary input is effected by synapses formed by the posterior processes of the bipolar papillary neurons whose cell hody locations have been described. The entry of these fibers into the ring is shown in the three parts of figure 28. In figure 28A the subdorsal papillary fibers are grouped as a bundle outside of the circumferential fibers of the nerve ring, but nonetheless within the thin glial sheath formed by the four LSM pocket cell bodies. In figure 28B at the posterior edge of the ring they are seen to turn from a longitudinal to a radial dIrection and plunge into the center of the circumferential fibers. At the level of figure 28C, taken anterior to figure 28A, all fibers except for those of the 60- and C-cells have completed a U-turn (the subdorsal LSM and 3-fibers accomplish this by branching and by multipolarity respectively as described) and again travel longitudinally but in an anterior dIrection. Shortly anterior to the U-turn all fibers diverge separately. The fibers may travel as much as 90 circumferentially and have aggregations of vesicles in presynaptic swellings which are large compared to the diameters of other circumferential ring fibers (fig 33, ILR).
A sagittal section through the entire dorsal extremity of the nerve ring, which consists of only about 100 fiber profiles (fig 29), shows a complete absence of penetration by the thin glial cytoplasm into the neuropil. Some organization is apparent. Most prominent is the anterior muscle plate region already described. It consists of a highly branched tangle of outward directed processes of the muscle sheets and is separated from the remainder of the neuropil by a slightly widened extracellular space which is occasionally seen to be filled with a slightly electron dense material (fig 30). All chemical synapses between ring and muscle fibers occur at the periphery of this delimited region, and no ring fibers penetrate into it. Numerous gap junctions are observed among the muscle processes themselves within the plate (fig 26, inset). Also apparent is a grouping of smaller, vesicle free fibers in the anterior ring region. Serial section analysis reveals few synaptic contacts within this cord of small fibers. This is in marked contrast to the surrounding neuropil consisting of larger diameter fibers which are densely packed with vesicles and form numerous synapses.
Four morphologically distinct vesicle types have been observed (fig 31). Whether these can be broken down further on the basis of histochemical properties remains to be seen. Most numerous are the typical small clear round vesicles 40 nm in diameter (fig 31A). These are the only vesicles observed at the synaptic darkenings at the muscle plates, and are observed in the central processes of the bipolar papillary sensory neurons. Nearly as numerous are slightly larger but much more irregular clear vesicles 55 nm in diameter (fig 31B). Dense core vesicles 65 nm in diameter, often mixed with clear vesicles, occur more infrequently and are associated particularly with fibers entering as part of the amphidial connective (fig 31C), never with papillary sensory fibers. Finally, we have observed that there are only two fibers, one on either side of the ventral midline, which possess neurosecretory-like vesicles of very large but non-uniform diameter (fig. 31D). The terminal swellings of these fibers occur just posterior to the ventral muscle plate and are connected to cell bodies in the region of tile ventral ganglion.
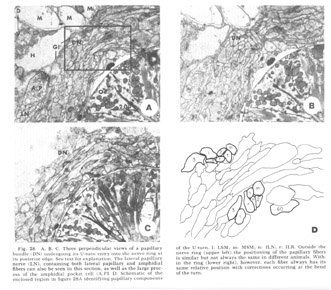 Figure 28. A, B, C. Three perpendicular views of a papillary bundle (DN) undergoing its U-turn entry into the nerve ring at its posterior edge. See text for explanation. The lateral papillary nerve (LN), containing both lateral papillary and amphidial fibers can also be seen in this section, as well as the large process of the amphidial pocket cell (A.P1 D. Schematic of the enclosed region in figure 28A identifying papillary components of the U-turn. l: LSM; m: MSM; n: ILN; r: ILR. Outside the nerve ring (upper left) the positioning of the papillary fibers is similar but not always the same in different animals. Within the ring (lower right), however, each fiber always has its same relative position with corrections occurring at the bend of the turn.
Figure 28. A, B, C. Three perpendicular views of a papillary bundle (DN) undergoing its U-turn entry into the nerve ring at its posterior edge. See text for explanation. The lateral papillary nerve (LN), containing both lateral papillary and amphidial fibers can also be seen in this section, as well as the large process of the amphidial pocket cell (A.P1 D. Schematic of the enclosed region in figure 28A identifying papillary components of the U-turn. l: LSM; m: MSM; n: ILN; r: ILR. Outside the nerve ring (upper left) the positioning of the papillary fibers is similar but not always the same in different animals. Within the ring (lower right), however, each fiber always has its same relative position with corrections occurring at the bend of the turn.
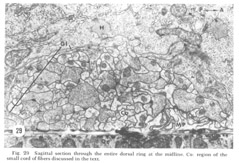 Figure 29. Sagittal section through the entire dorsal ring at the midline. Co: region of the small cord of fibers discussed in the text.
Figure 29. Sagittal section through the entire dorsal ring at the midline. Co: region of the small cord of fibers discussed in the text.
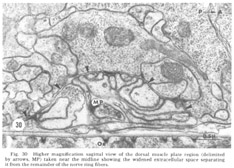 Figure 30. Higher magnification sagittal view of the dorsal muscle plate region (delimited by arrows, MP) taken near the midline showing the widened extracellular space separating it from the remainder of the nerve ring fibers.
Figure 30. Higher magnification sagittal view of the dorsal muscle plate region (delimited by arrows, MP) taken near the midline showing the widened extracellular space separating it from the remainder of the nerve ring fibers.
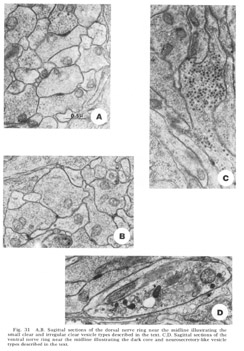 Figure 31. A, B. Sagittal sections of the dorsal nerve ring near the midline illustrating the small clear and irregular clear vesicle types described in the text. C, D. Sagittal sections of the ventral nerve ring near the midline illustrating the dark core and neurosecretory-like vesicle types described in the text.
Figure 31. A, B. Sagittal sections of the dorsal nerve ring near the midline illustrating the small clear and irregular clear vesicle types described in the text. C, D. Sagittal sections of the ventral nerve ring near the midline illustrating the dark core and neurosecretory-like vesicle types described in the text.
Figure 32. A. Point-type synapses from an unidenrified neuron onto an identified cell of the region of the ventrall ganglion. Inset: view of the same identified synapse taken in a perpendicular direction. N: nucleus of the postsynaptic cell. B. Serial point-synapse onto the dorsal muscle plate from unidentified fibers. C. Gap junction contact between two fibers of the irregular clear vesicle type in the dorsal ring. Inset: Higher magnification view of the same contact. D. A long en-passant synapse from an unidentified fiber onto several muscle sheet processes in the dorsal muscle plate.
By far the most common type of synaptic contacts are mediated by the so-called point synapses (fig 32A,B) which occur along the length of fibers and are not necessarily associated with a swelling of the presynaptic fiber. These are characterized by a very electron-dense presynaptic band usually packed with vesicles and forming a distinct convex protrusion from the presynaplic neuron. That the synapses are in fact highly localized can be seen in the two parts of figure 32A, which shows the same identified axosomatic synapses as cut in two perpendicular directions in different animals. Serial section analysis of junctions of this type in other regions of the neuropil has similarly verified their limited extend. Occasionally, both within the ring and at the muscle plate (fig. 32D), longer enpassant synapses without a membrane convexity are observed, although until preparative techniques have improved we cannot rule out the possibility that they are a series of point synapses. Both of these synapse types have the small clear round vesicles on the presynaptic side. Gap junctlon contacts are frequently seen (fig 32C). Invariably at least one, and usually both, of the participating fibers are of the irregular clear vesicle type. These fibers have never been observed to be presynaptic with a point synapse.
In addition to the highly unusual axosomatic synapse, we have also identified sensory motor synapse, which is of the point type, between the ILR fiber and the MPL muscle process (fig 33). This occurs not at the usual interior muscle plate region, but much more posteriorly, just after the entry of this muscle fiber into the ring. It is accompanied by a very large and terminal swelling of the ILR fiber which is nearly completely packed with vesicles. Serial section analysis of a number of these junctions has shown that they are composed of two point synapses, one slightly anterior to the other.
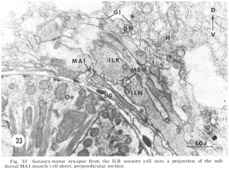 Figure 33. Sensory-motor synapses from the ILR sensory cell onto a projection of the sub-dorsal MA1 muscle cell sheet, perpendicular section.
Figure 33. Sensory-motor synapses from the ILR sensory cell onto a projection of the sub-dorsal MA1 muscle cell sheet, perpendicular section.
Discussion
In a simple CNS such as that of C. elegans the question of symmetry is of considerable interest. Superficially there is a hexagonal regularity to the cephalic region, as seen in the six-fold repeated gross lip structure and the identity of their internal labial papillae. More internally there is a four-fold regularity, as reflected by the identity of the submedial sensory structures and the positioning of the muscle cells within the four cephalic muscle bundles. Within the CNS itself, however, only a bilateral symmetry remains, but to which there are reproducible exceptions. In the anterior ventral ganglion (fig 18) for example, we have identified several asymmetrically located cells which as yet are incompletely characterized. Also, the multipolar DM cell sends a single process asymmetrically to the dorsal cord, itself asymmetrically constructed and asymmetrically innervating the cephalic musculature (fig. 25). Our observations thus support the conclusion of Schuurmans-Stekhoven ('37) that nematodes are constructed with fundamentally bilateral symmetry, but also show that at a fine structural level this is not perfect.
Of the possible function of the various anterior sensory receptors not a great deal can be said on the basis of morphology alone. Certainly the opening of the amphidial pouch to the external milieu and its content of an inhomogeneous matrix surrounding the dendrites strongly suggest a chemosensory function. The ciliary structure of the terminal amphidial neurons is also consistent with this conclusion (Han- sen and Heumann, '71).
The amphidial fine structure in C. elegans, not surprisingly, shows many gross similarities with that of the few other species which have been studied by electron microscopy, differences occurring mainly in details. The homolog of our pocket cell with its gland-like inclusions seems to be universal. McLaren ('70, '72) also finds the analog of our cap cell which completely surrounds the distal end of the pocket cell, extends into the amphidial pore and to the cuticle, and seals on itself with a very long longitudinal desmosome. Microtubules within the cilia have been found to show varying patterns of organization, from our 9 x 2 + m, to 8 x 2 + m in M. incognita (Baldwin and Hirschmann, '73), to a semi-regular pattern in D. immitis (Kozek, '71), to a complete lack of pattern in D. viteae (McLaren, '70, '72). The rudimentary rootlets which we invariably observe in longitudinal sections are variously described as occasionally present or missing. No distinct basal bodies have ever been described. Baldwin and Hirschmann describe some of the amphidial dendrites as leaving the pouch distally and migrating to the other lips [Because of this papillary organs are identified by the positional terminology of Goldschmidt ('03), Schuurmans-Steckhoven ('37), and Chitwood and Chitwood ('50) in order to avoid implications of homology suggested by the more commonly used terminology of de Coninck ('50)]. In C. elegans we see these as filamentous extensions of the dendrites contained completely within the pocket cell. Binary branching of some of the dendrites within the pouch is also mentioned, and dendrites are always described as ending at various levels and being surrounded within the pouch by an amorphous electron dense material. The presence of vesicles at the point of entry of the amphidial dendrites into the pocket cell has also been noted in D. viteae (McLaren, '70, '72), and the desmosomal junction with the pocket cell at this point has been shown in D. immitis (Kozek, '71). The terminal opening of the internal labial papillary organ presents a problem in trying to deduce a possible function solely on the basis of morphological evidence, On the one hand, the structure and configuration of the cilia and associated cells produce a sensilium similar in appearance to that of a chordotonal organ, responsive to motion parallel to its axis, described for scolopedia in crustacean stretch receptors and in insects (Mill and Lowe, '71; Whitear, '62; Howse, '68). Although the characteristic and presumably functionally important scolopale is absent, there is a cuticular darkening within the protrusion (fig. 5A) which, if rigid, could serve the same purported function. The similarity is further observed in the structural differences between the two dendrites, one resembling the ciliary type with along rootlet and a more or less organized array of microtubules, the other the paraciliary type in which the rootlet is short and illdefined and the ciliary microtubules are not as regularly arrayed. As in the Johnson's organ of Chrysopa (Schmidt, '69), it is this latter cilium which protrudes slightly from the cuticular opening, whereas the former ends somewhat more proximally. The opening does not immediately disqualify this organ as a pure mechano-receptor since such openings have been described in association with the company form sensilia in insects (e.g, Schmidt and Gnatzy, '71), in which case they are interpreted as an artifact of molting, In addition, the small diameter of the IL ciliary endings and their basically 9 x 2 + O microtubular arrangement is quite different from the larger diameter of the amphidial cilia and of the cilia of the phasmids (D. Hall, unpublished observations) and their 9 x 2 + m microtubular pattern. On the other hand, the fact that the dendrites do have direct access to the external milieu through a very narrow cuticular channel does raise the possibility of either a bimodal function, as in insect contact chemoreceptors, or a purely chemosensory function.
It is easier to exclude a possible chemosensory function for the MSM, LSM, and VL papillae and the deirids, since they possess no cuticular opening. In addition, their inclusions of very electron dense bodies at the dendritic terminations are much like similar specializations in insect mechanoreceptors. Of these inclusions, only those of the LSM organs have a close homolog in insects with the very common tubular body structure of campaniform sensilia originally described by Thurm and linked experimentally with detection of motion perpendicular to the dendritic axis (Thurm, '64).
The often striking similarities in the basic construction of the nervous systems of C. elegans and Ascaris have been pointed out in the description as they occurred. That these exist in such two disparate forms at opposite ends of the size and habitat scale is a validation of Chitwood's hypothesis that the nemic nervous system is conservative (Chitwood and Wehr, '34). In spite of the fact that Hyman stated "the greatest divergences from the usual plan are seen in Ascaris, which is also the form in which the nervous system has been most thoroughly studied" (Hyman, '51), these similarities clearly point out that different superficial structures may belie a unity on a much more fundamental level. Table 1 lists the papillary sensory cells which we have identified and, for comparison, those identified by Goldschmidt ('03, '08). It can be seen that the correspondence as to cell numbers is close but not identical. Most of our numbered cells have exceedingly fine fibers in the anterior part of C. elegans. Thus despite the size difference between it and Ascaris it is possible that some fibers could have been overlooked in a light microscopic study, even if the animals were constructed identically. Cell bodies were unlikely to be missed in the earlier study, however, so it seems reasonable to conclude, especially in light of the markedly different aggregations of cells into ganglia in Ascaris and in C. elegans, that there are in fact differences in the nervous systems of these two nematodes. A major disagreement with the earlier light microscopic work has been the finding that all sensory fibers of C. elegans maintain their discreteness throughout the neuropil and that they do not, as Goldschmidt ('08) stated for Ascaris sensory neurons, anastamose with one another after entering the nerve ring.
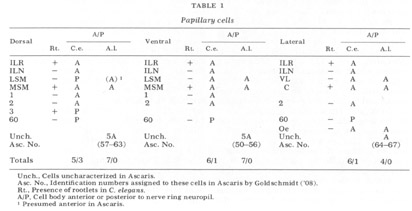
The cell body positions of all neurons, in particular the sensory neurons, have been found to be highly reproducible in different animals. Figure 18 shows this for two of the animals which we have studied extensively, and these are by no means exceptional. The only other investigation of comparable extent in a different invertebrate, the rotifer Asplanchna brightwelli, (Ware, '71) has likewise shown that every cell body is unique and has a homolog, both in position and in detailed ultrastructural anatomy, in all cases. This poses an interesting developmental problem which might well have been solved differently in different classes of animals. For those where cell economy is of primary importance, the nervous system must be constructed exactly if the species is to survive. For higher animals, where there is evidence of a high degree of redundancy in cell type; it may be that proper function can be achieved through an overall averaging process of many elements which are connected only approximately but into appropriate "fields" of influence. Consequently it is anticipated that the study of deviations from a normal structure in simple animals may reveal developmental principles which are obscured in higher, perhaps less well specified, systems.
The cephalic musculature has been shown to divide itself into four submedial quadrants, all of which are symmetrical in structure, The muscle innervation of the nerve ring has been found to be highly reproducible from animal to animal and, except for the possible detailed terminal arrangements which have not been investigated, similar for each quadrant. The peculiarity of the nematode muscle innervation has been shown to possess another unusual feature, namely that the muscle processes assume the shape of broad sheets within the central neuropil. What functional or developmental advantage this pattern possesses over the more commonly observed one of multiple branchings is unknown. The possibility of an interesting control mechanism arises in the different types of innervation of the four more anterior and the four more posterior muscle cells of each cephalic quadrant. The anterior four are innervated only by the ring, with very broad bellies present immediately before their sheets. The posterior four, on the other hand, have very slender processes, about 350 nm in thickness, interposed between their bellies and their sheets within the ring. They are also innervated by their respective medial cords, where much broader entry processes are present (fig, 25). In the event that the thin connectives to the ring have a relatively impared function in the well fed adult animal as we have examined it, one may well ask as to their significance. Only further examination of animals in various states of nutrition or in different stages of development will indicate whether the animal can perhaps utilize this bimodal innervation of the posterior cephalic musculature as a switch mechanism, that is, to alter the posterior cephalic muscle activity by alternating between pure cord and ring plus cord control.
There is no existing literature which deals extensively with the neuropil of the nematode CNS. This is unfortunate because, since the light microscopic work of Goldschmidt ('08) describing an apparent total anastamosing of fibers, there has been the tendency to think uncritically of the nematode nervous system as something "special," not conforming to the normal structural-functional pattern of other animals which have been investigated by modern electron microscopic techniques. At least in the case of the anterior sensory fibers, in contrast to the statement of Goldschmidt ('08), we have found this not to be the case. The nematode nervous system does possess one unique feature among the invertebrates in that it is also the location of the neuromuscular synapses. These contacts occur on branches of the muscle cells which are sent centrally to the motoneurons, rather than on branches of motoneurons which are sent peripherally to the muscle cells, It has been found that the synapses to the cephalic musculature occur largely at the borders of four delimited capsules, two lateral and two medial, within the nerve ring which we have called muscle plate regions. These regions contain only muscle cell processes, which are observed to have many gap junctions among themselves. A possible interpretation for the function of these junctions will have to await a determination of which muscle cells are coupled by them in all four quadrants as well as a description of any neighboring chemical synapses which occur on one or the other of the participating muscle processes. They cannot mediate a complete coupling, for the cephalic region of C. elegans is capable of very tight bending motions in all directions. At present all that can be said is that these junctions are numerous, cover large areas of the muscle processes within the plate, and in some cases have been found between homologous muscles subdorsally and subventrally. In the neuropil external to the muscle plates we have found a rather common variety of vesicles and synapses, though the presence of large numbers of gap junctions involving fibers containing irregularly shaped clear vesicles may perhaps prove unusual. Certainly a much clearer picture of the exact distribution and possible functions of these synaptic interactions will emerge from histochemical and connectivity analyses. In addition to the commonly observed structures, we have identified an unusual chemical synapse type, of the axosomatic variety, repeatedly observed on the same two bilaterally paired cells of the ventral ganglion (fig. 32A).
Our investigation has also added another animal to the list of those which possess the presumed intermediate in the evolutionary development of more complex nervous systems (Parker, '19), the sensory-motor cell (fig. 33). Doubtless other examples will be found as more systematic investigations are carried out in other invertebrates. Only two other instances of this have been shown. In Aplysia californica, Coggeshall ('71) described a cell containing large (120 nm) electron dense granules in the secretory epithelium of the accessory genital mass. The ciliated end of this cell, in which no rootlet was observed, extends into the lumen of the oviduct. The basal portion, which has granule-filled swellings, is found directly under large secretory cells. Although no membrane specializations resembling classical synapses were found, accumulations of vesicles were observed adjacent to the presumed post-synaptic effector cell. In Hydra littoralis, Westfall ('73) described a neuron which has at one end a cilium with two perpendicular centrioles and a faintly appearing striated rootlet. Both its cell body and neurite processes contain 100 nm dense core vesicles and many localized membrane specializations without swellings, taken to indicate areas of synaptic contact, opposite several different cell types. In contrast to these two examples, the ciliated sensory-motor cell of C. elegans has been found to have a typical presynaptic swelling filled with small clear synaptic vesicles and a membrane specialization adjacent to an identified muscle cell not unlike that found at other synaptic sites, both neuromuscular and neuroneuronal.
The description of the CNS of C. elegans presented here has concentrated on the gross organization of its main mass, the nerve ring, in relation to the primary sensory input and cephalic muscle motor output. Clearly much more work would be required to establish the anatomical basis for behavioral patterns of the animal in even the crudest sense. The diffuseness of the nervous system as a whole, composed as it is of numerous peripheral cords which themselves innervate muscles, provides a formidable challenge for serial section analysis. Nevertheless the results presented provide a fine structural basis with which one can begin to form associations of observed behavioral abnormalities with alterations in the central controlling nervous tissue, and lay the groundwork for electron microscopic examination of detailed connectivities in a central neuropil.
Acknowledgments
Thanks is given to the Environmental Sciences Department, California Institute of Technology, for extensive use of, and permission to modify, their Zeiss EM9 electron microscope, and to Chris Irving for illustrations.
This research was supported by a Neurosciences Study Grant from the Sloan Foundation and by U. S. Public Health Service grants NS-09654 and NS-10628.
|
