|
Studies on the Development and Organisation of the Nervous System of Caenorhabditis elegans
Part I - The Outgrowth of Nerve Processes in the Embryo
see also: Part II - The Organisation of the Adult Nerve Ring
Richard Michael Durbin
Thesis published 1987
Preface - Summary - Part I - Part II - Appendix - Conclusion - References - PDF
Preface
April, 1987
The work described in this dissertation was carried out between January 1984 and March 1987 at the MRC Laboratory of Molecular Biology, Cambridge. As described in the summary, two different approaches were used in this work, and the main body of the dissertation is split into two parts, each with its own introduction. However the introduction to the first part provides much of the general background. There is a final conclusion which considers both parts in a broader setting.
It is customary to list a long series of acknowledgements somewhere in the preface to a dissertation. I have derived enormous personal and scientific benefit from my time spent at the Laboratory of Molecular Biology, both from the people who work here and the environment that they have created. I am only going to thank personally two people, my supervisor John White, to whom I owe so much that it would be pointless to try to encapsulate it, and Nichol Thomson, who does all the serial sectioning of C. elegans at the MRC with remarkable consistency, and ultimately without whom none of this work would have been possible. I would also like to thank the Medical Research Council for a Training Award, and King’s College for a Research Fellowship.
With the exception of the technical serial sectioning for the first part, this dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration. No part of this dissertation has been or is being submitted to any other University.
May 18, 2001
This thesis was written in 1987 and has not been updated since. The discussion of related literature is therefore severely out of date. It is being made available because it provides the primary source for quite a lot of material that was not published elsewhere, in particular concerning the early outgrowth of neurons in the ventral and dorsal cords.
Summary
The nematode Caenorhabditis elegans is a small invertebrate whose nervous system, general anatomy, and normal development are all (comparatively) extremely simple and reproducible, and have all been well characterised. This dissertation describes work based on two different approaches to the study of the control of neural development in C. elegans.
In the first part the course of neural outgrowth in the region of the ventral nerve cord was followed from electron microscope reconstructions of a series of fixed embryos. Following this, neurons whose processes grew out early were removed by laser ablation of their parent cells and the effect on subsequent nerve outgrowth was assayed by electron microscope reconstruction. The first process to grow along the ventral cord is that of AVG, and its presence is required for the normal, highly asymmetrical structure of the cord. Two more examples of dependancy on particular nerve processes for correct guidance can be deduced from experiments in which cells at the back of the animal were removed. The observations of normal development and the ablation experiments can in some cases be related to defects seen in uncoordinated mutants with defective nerve process organisation.
The second approach was to establish and analyse a computer data base containing all the synaptic connectivity data obtained by White et al. (1986), who reconstructed at an electron microscope level the entire central nervous system regions of two C. elegans specimens. Since the circuitry is highly reproducible, comparisons of connections between equivalent pairs of cells can be used to infer properties of synapse formation. Overall, the C. elegans circuitry is anatomically highly directional, and what little chemical synaptic feedback that is seen is mostly part of reciprocal synaptic connections. There is also evidence for physical organisation of the nerve processes in subbundles of the main process tract in the central nervous system.
WA editors' note: Here is a list to help the reader match the embryos analyzed by Richard Durbin to the image files hosted on WormImage.
Embryo called "A" in thesis = RDA on WormImage
Embryo called "B" in thesis = RDB on WormImage
Not featured in thesis
but RDC embyro is on WormImage
Embryo called "C" in thesis = RDD on WormImage
Embryo called "D" in thesis = RDE on WormImage
Embryo "AVG ablated" in thesis = embryo_G2_(AVG ablated)
Embryo "PVQL ablated" in thesis = either embryo_QA_(PVQL ablated) or embryo_QB(PVQL ablated)
Part I - The Outgrowth of Nerve Processes in the Embryo
Chapter 1: Introduction
1. A review of neural guidance
2. The C. elegans nervous system
Chapter 2: Materials and Methods
1. C. elegans neuronal nomenclature
2. Electron microscopy
3. Ablations
4. Reconstruction
5. Staging of reconstructed embryos
6. Identification of neurons
Chapter 3: The Pattern of Outgrown in Normal Embryos
1. Morphology of grown cones
2. The attachment substrate for growth cones
3. AVG pioneers the ventral cord
4. Motor neurons
5. Later ventral cord interneurons
6. Decussation in the preanal and retrovesicular ganglia
7. Growth cone insertions into other cells
Chapter 4: Laser Ablation Experiments
1. AVG
2. DD3/5
3. PVP and PVQ
4. DVC
Chapter 5: Discussion
1. Reliability
2. The asymmetry of the ventral cord
3. Motor neuron outgrowth and formation of the dorsal cord
4. Discussion
5. Selective fasiculation
6. Conclusion
Chapter 1: Introduction
1.1 A review of neural guidance
1.1.1 Growth cones in vitro
1.1.2 Studies in vivo
1.1.3 Directional and tropic effects
1.1.4 Fasciculation of nerve processes
1.1.5 Pioneers and specific fasciculation
1.1.6 Interactions with non-neuronal surfaces
1.1.7 Summary
1.2 The C. elegans nervous system
The first part of this dissertation describes an investigation into the outgrowth of nerve processes in the region of the ventral nerve cord of C. elegans during embryonic development. The course of normal development was deduced from serial section reconstructions of a set of embryos fixed at different stages. Then laser ablation experiments were performed to remove specific neurons whose processes grew out during these early stages, in order to test whether the presence of these processes was necessary for correct subsequent development of the nervous system. Chapter 2 gives materials and methods. The observations from the wild type reconstructions are given in Chapter 3 and the results of the ablation experiments are described in Chapter 4. The two sets of results are discussed together in Chapter 5. There are no previous direct results on the course of neural outgrowth in C. elegans, although disruption of the final arrangement of nerve processes has been observed in mutants (Hedgecock et al., 1985) and animals in which laser damage has prevented nerve cell migration (Chalfie et al., 1983). Below I first review previous work in other systems on neural guidance, and then give an introduction to C. elegans and its nervous system.
1.1 A review of neural guidance
The building of a nervous system during development can be divided into three phases: the generation of the correct cells in the correct places, the outgrowth of nerve processes, and the formation of synapses. All of these phases show a high degree of specificity, which means that a large amount of information must be expressed by mechanisms that on the whole we do not yet understand, but would like to. In some ways the second phase, that of process outgrowth is the most clearly defined. This is because all neural branching structures are a consequence of a single phenomenon, the migration of growth cones during development, a truth which Cajal saw early and fought hard for (Hamburger, 1981), and which led Harrison to develop the first tissue culture techniques in order to follow outgrowing neurites directly (Harrison, 1910).
A growth cone is a specialised structure at the tip of any growing neurite that migrates through the animal, spinning out the nerve process behind it. This is not the only means by which nerve processes can be lengthened, since change in size and shape of the animal is matched by addition of new material to already existing processes. In many cases most of the length of nerve fibres is created in this way, but it is almost entirely passive, having at most a very small effect on the layout of the neurons axonal structure. For instance, most nerve processes grow along the ventral cord of C. elegans when it is only around 100 microns long, a tenth of its final length. However some changes in overall structure do occur by intercalary insertion; an example is the conversion of an initially bipolar cell to one that is pseudo-monopolar, by retraction of the cell body away from the branch (Kuwada, 1986 and with ventral nerve cord motor neurons in C. elegans). Such small alterations during subsequent development emphasise the importance of looking at outgrowth as it takes place, rather than making inferences from the finished pattern.
1.1.1 Growth cones in vitro
Growth cones are generally spread out, lamellar structures, which often extend fine microspikes, or filopodia (Letourneau, 1983). They mover over surfaces and as they move the various lamellar and filopodial extensions are retracted and new ones are extended out, so that the overall shape is continually changing (Bray and Chapman, 1985). It is easy to study growth cone migration in vitro using cultured neurons and a wide range of factors that affect migration have been observed. In order for motion to take place it appears that fairly tight adhesion to the substrate is necessary (Bray, 1979), and this leads immediately to the idea that differential adhesion may be important for growth cone guidance. Letourneau (1980) has shown that growth cones do indeed tend to grow along regions of higher adversity when faced with a choice in vitro. Although this may support the common suggestion that growth cones may in many cases be guided up an adhesive gradient in vivo (Nardi, 1983, Berlot and Goodman, 1984), it does not directly address that proposal, and there are several severe problems with the idea. Growth cones show different morphologies when migrating on artificial surfaces of different adhesivities, but even though the range of morphologies seen on different neurites in vivo is vast, any one growth cone does not change shape as it migrates over a uniform surface. In addition the strength of adhesivity would have to increase exponentially, which would require an excessive magnitude range of adhesivity for a gradient of any substantial length. In fact growth cones in culture tend to grow in straight lines anyway, only changing directions when they branch. Based on an elegant combination of observations and experiments Bray has suggested that the neurite leaving the back of the growth cone exerts a tension, and that the growth cone always grows away from the source of tension (Bray, 1979). If the angle of the neurite is altered then the direction of growth coordinately changes, and if the tension is relaxed, by for example cutting the neurite, then the growth cones divides in two, the two halves growing off in opposite directions and exerting tension against each other. Together these results suggest that direction changes and branches may occur in vivo either where a path of higher adversity is crossed, or possibly at a point where the growth cone becomes tethered, so that growth in the new direction can pull against something.
Although there is a strong tendency to think of attractive forces on growth cones as being the principle tools of guidance control, it is equally possible for repulsive forces to be influential, and there are several examples that are known. There is a highly selective inhibitory effect when the neurotransmitter 5-HT is released from a micropipette near the advancing growth cone of an identified cell from the mollusc, Helisoma (Haydon et al., 1984). This has been proposed to have developmental significance in the detailed development of the Helisoma buccal ganglion (Meinertzhagen, 1985). A retraction of the growth cone in vitro is also seen when retinal and sympathetic axons meet each other in culture (Bray et al., 1980, Kampfhammer et al., 1986). Although retinal growth cones will cross retinal axons, and sympathetic growth cones will cross sympathetic axons, when one meets the other it shrinks back and withdraws its filopodial and lamellar extensions. Similar avoidance behaviour between different neurites of the same neuron could possibly explain the marvellous space filling, non-overlapping properties of many neurons' dendritic or axonal arborisations. Experimental evidence for such avoidance has been provided by studies of single sensory neurons in the leech, which fill a planar surface from several points in an apparently self-competitive fashion (Kramer and Stent, 1985). As yet there is no experimental evidence of such mechanisms acting between different neurons in vivo, but there are several cases in C. elegans where neurons abut against but do not overlap other members of their own classes; often there is a gap junction between the two abutting processes (see Chapter 7).
1.1.2 Studies in vivo
It is convenient to make a distinction between directional, tropic influences on neural guidance and spatially restricted, contact mediated influences. Both appear to play an important part. To oversimplify the situation, tropic influences are directionally constraining, while differential adhesivities are spatially constraining. There is also a division between specific and nonspecific factors. By nonspecific factors I mean those that would influence any of a large range of different neurons. Neither of these divisions is totally sharp, and in particular specificity is clearly a graded phenomenon.
The classical example of a non-specific factor would be a gradient of positional information (Wolpert, 1971), probably some chemical or surface marker, and the classical experimental system where there is evidence for such a gradient in neural development is in the establishment of a topographical mapping from the retina onto the optic tectum of lower vertebrates. A series of experiments in which an ordered mapping reformed after parts of the retina and/or tectum were removed or grafted back in abnormal orientations suggested that the original chemo-affinity hypothesis of Sperry (1963), which proposed specific matching between corresponding sectors of the retina and tectum, was incorrect (see Gaze, 1970). More recently Bonhoeffer and Huf (1982) have shown using an in vitro axon growth choice assay that there is a gradient of affinity for temporal axons across the surface of the tectum, with highest affinity for the rostral part of the tectum, which is their normal target. Progressively more nasal axons show less specificity. The overall effect of these affinities would then be established by competition. There are many other proposed sources of information for the retino-tectal system, some also driven by competition (e.g. Willshaw and von der Malsburg, 1979).
However the situation during creation of the retino-tectal map on the surface of the tectum is different from the early outgrowth of processes that concerns the study of embryonic C. elegans outgrowth in this dissertation, since the axons have already reached their target tissue and are finding the correct place on it amongst a group of equivalent cells. For the rest of this review I will focus on the pathfinding properties of growth cones necessary to find their targets from the cell bodies, rather than the final stage as discussed here.
1.1.3 Directional and tropic effects
A very different function of a gradient is to specify a direction up which axons can travel. There are several examples where a general attraction that is not path specific has been indicated experimentally. Harris (1980) has shown this type of effect using the same retino-tectal projection in Xenopus mentioned above as an experimental system, but at the earlier stage of development where the optic tract must be formed. Before axon outgrowth he implanted whole eye primordia into abnormal places in the brain, after which in most cases the retinal axons grew out and took a nearly direct route to the tectum, usually via a pathway totally different to the one they normally follow. If the implant was sufficiently caudal then the retinal processes ran instead down the spinal cord, in a particular dorsolateral tract, reproducing previous observations that this part of the spinal cord attracted displaced retinal axons (Constantine-Paton and Capranica, 1976). These results suggest that there is a general attraction of retinal axons to their target, and that this acts over a fairly wide zone, but that the mechanism may not be uniquely used for retino-tectal pathfinding; in the spinal cord, outside the normal range of retinal axons the same attraction system may be used for another set of processes.
A more specific attraction of neurons to their targets has been observed in the vertebrate peripheral nervous system (PNS). Lance-Jones and Landmesser (1981) showed that after a short piece of chick neural tube was reversed the motor neurons till largely found a way to the correct target muscles, crossing over each other on the way. However if the displacement is too great then they often grow to inappropriate muscles (ibid. and Summerbell and Stirling, 1981). Again this influence appears to be over a longer range than the reach of the filopodia, though still reasonably localised (Landmesser, 1984). There are also indications in the insect PNS that after the more specific cues are removed there is still a tendency for sensory neurons to grow proximally towards the central nervous system (CNS), even along abnormal routes (Berlot and Goodman, 1984, Nardi, 1983).
One suggestion of a possible agent involved in the general attraction of a whole class of nerve fibres is nerve growth factor (NGF). Sympathetic fibres grow over abnormal territory towards a site of NGF injection in vivo (Gunderson and Barrett, 1980). However in both cases the amounts applied are much larger than the observed natural levels; NGF is much better known as a trophic agent necessary for neuron survival and a general promoter of neuron outgrowth, and the directional effect may be a subsidiary non-physiological consequence of an overdose of these other behaviours. In a careful set of experiments with explants from embryonic mouse trigeminal ganglia and their target tissue, maxiliary epithelium, Lumsden and Davies (1983, 1986) have shown a clear directional tropic attraction of trigeminal fibres to their target. This is diffusible through the colagen matrix in which the explants sit and the axons grow, and is separable from NGF, which appears to act later in development to preserve the connection. It also has no effect on axons from comparable neighbouring ganglia. Lumsden and Davies argue that NGF is active on too many cell types to be sensible as a tropic agent. However it might be countered that a general tendency for sympathetic axons to grow towards the periphery could be useful.
All these results suggest that there may be general directional (often homing) guidance mechanisms that are not restricted to specific pathways, and apply to fairly broad classes of neurons. Interestingly the range of all the attractions is approximately the same, of the order of a few hundred microns. In cases that are more specific, such as the chick motor neuron guidance, the absolute size of the embryo is larger. Such distances correspond to a fairly small number of growth cone extensions, suggesting that a growth cone could detect a gradient on this scale. Since some specificity is involved and the directions of different sets of fibres can cross (as in the chick limb motor neuron experiments), it seems unlikely that a single gradient, such as a general adhesive gradient, provides the best explanation for them. In at least one case (Lumsden and Davies) the substrate if artificial and the factor is diffusible.
Before automatically explaining any experiment indicating a directional effect by a gradient, it should be born in mind, however, that there are at least two other ways in which a polarity or directionality could be specified. The first is intrinsic to the neuron, simply by the orientation in which it was created by its final cell division. This may often be important for initiating process outgrowth in the correct direction (Jan et al., 1985). The second is by a repeated sequence of more than two signals, in which case the direction can be determined by inspecting neighbouring sequence elements, or equivalently by a moving wave of some signal. This type of signal can operate over very long distances if it is actively maintained, and is the method is slime mould aggregation (Gerisch, 1982).
1.1.4 Fasciculation of nerve processes
A different sort of nonspecific influence that is important for neuronal outgrowth is the strong tendency of growth cones to grow along other neurons, which leads to the fasciculation of nerve processes. This is clearly one of the most important factors determining the structure of the peripheral nervous system, which is made of nerve bundles, and where closely studied it has also been seen to be important in the early developing central nervous system at stages where processes are not dense (e.g. the insect CNS, Bate and Grunewald, 1981, Goodman et al., 1982). This has been seen by immunofluorescence to be expressed on many neuronal cell surfaces, and also on various epithelial and glial cells (Silver and Rutishauser, 1984). It has been claimed that the modulation of a single molecule such as NCAM could account for a very large proportion of the control of neural outgrowth (Edelman, 1983), but this appears unlikely because of the degree of specificity seen in many different but often adjacent and simultaneous interactions. However there is a large part to be played by fairly non-specific adhesion.
Almost a direct consequence of general neuronal fasciculation is the concept of the preservation of order within nerve bundles by a process tending to stay stuck to its neighbours. Many nerve projections show a general topographic order preservation, both in the central and peripheral nervous system (e.g. the retinal-tectal and spinal cord projections) and a simple method of correct guidance may be to place neurons in positions corresponding to a topological map of their targets and then to preserve the relative spatial arrangement in the outgoing bundle of fibres and rely on non-specific cues to spread the projection onto the target tissue(s). In fish retino-tectal projections Scholes (1979) has shown that order is in general maintained, but that there is a zone of active reorganisation near the tectum, and in other cases where ordering has been observed an active mechanism for correcting the final projection has also been detected (e.g. Landmesser, 1984).
1.1.5 Pioneers and specific fasciculation
The observation that fasciculation is a significant factor led to a realisation of the importance of the first nerve pioneers to grow out, called "pioneers" by Harrison (1910) and to the suggestion that they may be specialised in order to be able to lay down new paths. The pioneers in a various part of different insect peripheral nervous systems have been studied first by Bate (1976a), and subsequently by many others (e.g. Ho and Goodman, 1982, Bentley and Keshishian, 1982, Blari and Palka, 1985, Jan et al. 1985). Although in certain cases outgrowing central neurons grow out over new territory (Ho and Goodman, 1982), the majority of nerve bundles are pioneered by peripheral sensory neurons that essentially always follow a series of other neuronal cell bodies spaced out at intervals on the way to the CNS. This observation led to the "guidepost" hypothesis, that there are a class of specified cells in the periphery that are guideposts (maybe all neurons) and that pioneer growth cones search for and grow towards the nearest guidepost cell within reach at each stage (Bentley and Keshishian, 1982). In this case it appears that no single pioneer is essential, since various cell removal experiments resulted in satisfactory correction or adaptation (Keshishian and Bentley), 1983, Blair and Palka, 1985).
Ho and Goodman (1982) argue for a certain degree of specificity of fasciculation in the grasshopper PNS, particularly for outward growing CNS axons which must choose branches at points where afferent fibres have converged. There appears to be a much greater amount of specificity in the grasshopper CNS. Here again the earliest pioneer fibres have been identified (Bate and Grunewald, 1981), and the subsequent outgrowth of certain identified neurons has been followed (Goodman et al., 1982). A large number of closely adjacent fascicles are established and growth cones often cross a number of them before fasciculating with a particular one. This has lead to the "labelled pathways" hypothesis (Ghysen and Jansen, 1979, Goodman et al., 1982), that the fascicles are differentially labelled by surface molecules and that growth cones are programmed to recognise a sequence of these labels and grow along them, thus defining a route through the developing nervous system. Ablations of neurons that generate the pathways for identified cells in this system have resulted in the stalling of growth cones (Raper et al., 1984, Bastiani et al., 1986). This contrasts with what has been seen in the PNS, and provides a genuine example of a specialised pioneer, whose presence is necessary for later axons to follow.
The chick PNS experiments described earlier provide another example of the requirement for a preexisting fascicle along which a subsequent neuron type will follow. In the experiments in which sections of neural tube, or limb buds, are displaced, sensory neurons that innervate muscle only follow the correct pathways to their muscles if the corresponding motor neurons do so (Honig et al., 1986). Furthermore, if instead of displacing motor neurons the whole motor neuron pool is removed before axon outgrowth, so that later there is no motor innervation of muscle, then there is effectively no sensory innervation of muscle either, and instead cutaneous sensory innervation is increased (Landmesser and Honig, 1986).
Therefore, in addition to the nonspecific general tropism and fasciculation that were discussed earlier, there is substantial evidence for specific interactions between neurons and bundles of other neurons with which they will fasciculate. In the case of the insect CNS the specificity appears to be almost certainly mediated by contact; not only are the differing choices too tightly packed for a longer range influence to be sufficiently selective, but there have also been seen in the electron microscope direct interactions of growth cone filopodia inserting themselves deep into the surfaces of cells they will eventually fasciculate with (Bastiani and Goodman, 1984). Monoclonal antibodies have recently been made that appear to recognise specific fascicles in the grasshopper CNS, and the growth cones that will join them (Harrelson et al., 1986). Interestingly in each case several different bundles stain with the same antibody. If the antigens are involved in determining fasciculation then this would be reminiscent of the observation with ectopic retinal implants that there seems to be an affinity of retinal axons for an abnormal target in the spinal cord, as well as the tectum.
1.1.6 Interactions with non-neuronal surfaces
Up until now the interactions between growth cones and their targets, or other neurons, have been stressed, but clearly their relationship to non-neuronal substrates may also be important, particularly for pioneer neurons. In various different situations growth cones have been proposed to migrate over basement membrane, glial cells, epithelial cells, and mesenchyme. One of the strong reasons for proposing basement membrane as a possible neuronal substrate is that both raw basement membrane and several purified basement membrane components, such as fibronectin and laminin, have been shown to provide good surfaces for outgrowth in vitro (Varon-van Evercooren et al., 1982). Also in vitro processes are often found growing in spaces adjacent to a limiting basement membrane (e.g. the CNS pioneers in the grasshopped, Bate and Grunewald, 1981, or the first fibres in the fish spinal cord, Kuwada et al., 1986). However this region almost always also contains a large number of glial processes, and at least in the case of retinal axons, the nerve fibres seem to be particularly strongly attached to these glial endfeet (Krayanek and Goldberg, 1981), which have been shown to stain early on for NCAM (Silver and Rutishauser, 1984). The ordered outgrowth of retinal axons can be disrupted by injection of anti-NCAM antibodies (ibid.). In addition Silver and Ogawa (1981) have shown that a preformed glial bridge is necessary and sufficient for growth of neocortial fibres across the corpus callosum.
On the basis of this type of observation, Singer et al. (1979) proposed the blueprint hypothesis, suggesting that there was a preformed meshwork of favoured pathways established on the glial and neuroepithelial external surface, which would channel growth cones in the same sort of way as Letourneau's adhesive grid in vitro (Letourneau, 1980). As with fasciculation, to which this type of concept is clearly related, non-neuronal blueprints could come in a complete range of specificities, from generally available for all axons to completely specific for a particular growth cone. In the case of the grasshopper CNS it has been possible to implicate a particular glial cell, the segment border cell, as determining the exit site for one of the main connectives to the periphery (Bastiani and Goodman, 1986). It effectively acts as a specific labelled pathway itself.
1.1.7 Summary
There is no case where the underlying mechanisms that control a nontrivial outgrowth pattern for a particular neuron or type of neuron have been determined in detail. One of the reasons for this is that we still know too little about the molecular and cellular basis of growth cone movement and guidance (Letourneau, 1983). On a larger scale, there are a number of experiments suggesting various sources of influence for process outgrowth. These experiments normally involve perturbation of particular factors in vivo and the results can sometimes be open to variable interpretation, depending on the hypotheses being addressed by the interpreter. One certain conclusion, however, is that a large range of different mechanisms can be used to influence neural guidance, usually in various combinations, and often in a redundant fashion. The information necessary for determining the outgrowth of any particular neuron will be expressed via a subset of these factors, the relevant subset probably differing in different stages of outgrowth.
Therefore the best that can be done at the general level is to identify the basic forms of the different types of relevant influence and interaction, and provide a list of tools that are available to whatever program controls development. In generating such a list I again restrict myself to outgrowth from the cell to the target, rather than interactions on the target tissue in which competition and neural activity may well play a part. With this restriction there currently seems to be evidence for the following list:
1. Much of the necessary organisation can be achieved by the initial positioning and orienting of the neurons.
2. There is a general tendency for axons to extend in straight lines unless otherwise influenced.
3. There can be local inhibitory influences on growth cone extension, either humoral or contact mediated.
4. Adhesion is clearly important for growth cone migration, and it seems likely that preformed generally adhesive pathways provide a set of preferred highways for processes to grow along.
5. Also in the realm of general adhesivity, there is a strong tendency for extending neurites to fasciculate together.
6. Both these last two influences can also act in a specific, as well as a non-specific, fashion, for example when a growth cone joins one particular fascicle out of several.
7. There can be a directional attraction of axons, normally from some fairly broad class of neurons, to some target or region, and this can function when a normal route is unavailable. At least in some cases this attraction is mediated by diffusible factors.
For those elements of the list where there is specificity, as in the last two cases, it seems that the same specificity mechanism may be used in more than one place.
Even if this list were complete, it would only provide a framework for two further lines of inquiry. The first is to search for the molecular and cellular mechanisms involved in each type of interaction, and the nature of their possible diversity and specificity. The second is to investigate how the consequent repertoire of available influences intricate outgrowth patterns for the huge variety of different neurons. One way to attack these problems is to choose an organism where the types of interaction involved and the different levels of specificity can be made as clear as possible, and then use the experimental power of molecular genetics as a technique to probe both the nature of the molecules concerned and the internal control structure of the genome. A good candidate for that organism is C. elegans.
So far in this introduction I have mixed examples from invertebrate and vertebrate model systems fairly freely, since many of the results can be directly compared, and it seems likely that factors which control growth cone guidance at the cellular level may well be analogous, if not identical, between even very widely diverged species. The significant difference between invertebrate and invertebrate nervous systems for the purposes of experimentation on axon guidance is that, in addition to in general containing orders of magnitude fewer cells than vertebrate ganglia, many and in some cases all, neurons in an invertebrate ganglion are reproducibly identifiable from one animal to the next. Often there will be only one or a small reproducible number of cells with any particular set of characteristics. Therefore repeatable experiments can be undertaken concerning a known individual neuron and the specific factors involved in controlling the outgrowth of its processes. C. elegans contains only 302 neurons altogether, all of which are identifiable, and for all of which the complete audit anatomy is known at the electron microscope level (White et al., 1986).
Finally, but not least importantly, we turn to the use of genetic techniques to study neural outgrowth. The primary reason for choosing C. elegans as a model organism for the study of neural development was not the simplicity of its nervous system, but that it is well suited to genetic analysis (Brenner, 1974). The reason that genetics has not been mentioned before this point is that, although it can provide an extremely powerful tool for studying biological function and control and has been extensively used to study neuronal cell determination (e.g. Lehmann et al., 1983, Hedgecock, 1985), it has as yet provided very little insight into neural guidance. In vertebrates a few known mutations affect neuronal branching patterns and guidance, such as mouse mutants weaver, staggerer and reeler, which affect the structure of various cell types in the cerebellum (Caviness and Rakic, 1978). In Drosophila there are several mutations that have been used as experimental tools to remove neurons, or produce them in abnormal places (e.g. the homeotic mutants, Palka, 1982) but the only published mutation that seems to directly affect neuronal guidance is bendless, in which one of the neuron types involved in the escape jump response fails to reach its target (Thomas and Wyman, 1982). However it is not known whether other processes are affected, nor is the wild type development of the particular neuron known. In fact the organism in which the greatest number of neural guidance specific mutants are known is C. elegans (Hedgecock et al., 1985, S. McIntire, J. White, E. Hedgecock, personal communications, discussed further in the next section). In addition to any intrinsic interest and possible significance, it was in order to provide the developmental framework for further characterisation of the molecular mechanisms involved in guidance via this genetic approach that the study described in this thesis was undertaken.
1.2 The C. elegans nervous system
C. elegans is a small nematode, or roundworm, approximately 1mm long in the adult form. It has a simple body structure and a small number of cells: 959 somatic cells including 302 neurons. Development from egg to fertile adult takes only three and a half days at room temperature. Wild type animals used in this study are isogenic, since the egglaying sex is a self-fertilising hermaphrodite, rather than a female, with the consequence that strains are normally propogated asexually, forming clones. Males occur naturally at low frequencies. Their hermaphroditism also facilitates genetic analysis, and many mutants have been studied. Together these facts make C. elegans a favourable model organism for the detailed study of development at the level of single cells, using both anatomical and genetic techniques, and it was chosen as such by Sydney Brenner (1974).
The life cycle consists of an embryonic stage, inside the egg, which takes about 16 hours, followed by four larval stages, named L1 to L4. The course of development is extremely reproducible. The pattern of cell divisions from the fertilised egg to the adult has been determined completely (Sulston and Horvitz, 1979, Kimble and Hirsh, 1979, Sulston et al., 1983) and is essentially invariant.
Not only are the pattern of cell division and the general body plan of C. elegans simple and reproducible at a cellular level, but so is its nervous system. The complete nervous system of the adult hermaphrodite has been reconstructed by White et al. (1986) from electron micrographs of serial thin sections. The neurons have simple branching structures, and both the dispositions of cell processes, and the connections they make, appear to be largely invariant between animals. They can be assigned to 118 different neuronal classes on the basis of morphology and synaptic connectivity (the system of nomenclature is described in Chapter 2). An overview of the nervous system of an L1 larva is shown in Figure 1.2. Its central processing region is a loop of neuropil around the pharynx, called the nerve ring, containing around 175 nerve processes. Running from this is a set of longitudinal process bundles that connect the ring to sensory receptors, the body motor nervous system, and several small ganglia in the tail. There are also circumferential commissures carrying processes from one longitudinal bundle to another. The most important of the longitudinal bundles is the ventral nerve cord, which runs from the retrovesicular ganglion (RVG) just behind the nerve ring to the preanal ganglion (PAG) at the beginning of the tail, and containing the motor neuron cell bodies for the body motor circuitry.
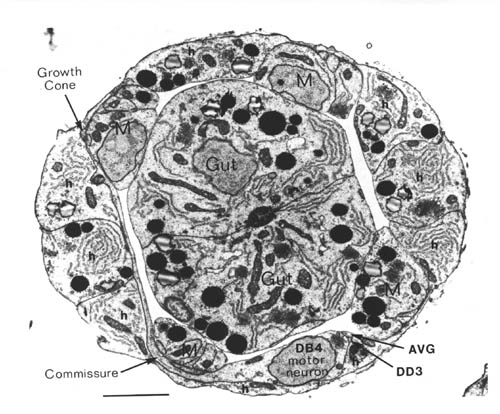 FIGURE 1.1. Transverse section of a 515 minute embryo (the C reconstruction of Chapter 3). The gut, muscle quadrants (M) and outer hypodermis (h) are all labelled. There are two nerve processes in the ventral nerve cord (AVG and DD3), and one motor neuron cell body (DB4). A left handed commissure is growing out from the DB4 cell body towards the dorsal hypodermis. In its growth cone can be seen a number of small vesicles. Scale bar is 2 microns.
FIGURE 1.1. Transverse section of a 515 minute embryo (the C reconstruction of Chapter 3). The gut, muscle quadrants (M) and outer hypodermis (h) are all labelled. There are two nerve processes in the ventral nerve cord (AVG and DD3), and one motor neuron cell body (DB4). A left handed commissure is growing out from the DB4 cell body towards the dorsal hypodermis. In its growth cone can be seen a number of small vesicles. Scale bar is 2 microns.
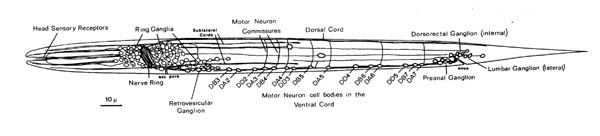 FIGURE 1.2. A general view of the L1 larva and its nervous system. All the neuronal cell bodies and process tracts behind the retrovesicular ganglion on the midline or the left side are shown. The main region of neuropil is the nerve ring, which is a loop around the pharynx. The ventral cord runs back from this and contains motor neuron cell bodies in addition to processes. Those ventral cord motor neurons that do not send a commissure around the left side of the body to the dorsal cord send one to the right side. There are four small tail ganglia: the preanal ganglion, the dorsorectal ganglion, and two lumbar ganglia, one on each side.
FIGURE 1.2. A general view of the L1 larva and its nervous system. All the neuronal cell bodies and process tracts behind the retrovesicular ganglion on the midline or the left side are shown. The main region of neuropil is the nerve ring, which is a loop around the pharynx. The ventral cord runs back from this and contains motor neuron cell bodies in addition to processes. Those ventral cord motor neurons that do not send a commissure around the left side of the body to the dorsal cord send one to the right side. There are four small tail ganglia: the preanal ganglion, the dorsorectal ganglion, and two lumbar ganglia, one on each side.
Nerve cells in C. elegans are small (less than 5 microns in diameter) and it is not currently practical to impale them with microelectrodes. However intracellular recording from selected neurons has been possible in the larger nematode, Ascaris lumbricoides. Attention has been focussed on the ventral cord motor circuitry (reviewed in Stretton et al., 1985), and the distribution of cell types seen there corresponds anatomically very closely to that in C. elegans.
Previous studies on neural process guidance in C. elegans have been restricted to examining the structure of the adult nervous system in both wild type animals and mutants in which processes go astray. White (1983) discusses some possible factors that may be important in neural guidance on the basis of the adult electron microscope reconstructions. Chapter 9 of this thesis also considers process placement in the nerve ring using data from the adult reconstructions. Several techniques (mostly unpublished) have been developed to visualise processes by light microscopy, and these have been used to screen mutants that have possible neural defects, such as uncoordinated mutants that do not move well. Hedgecock et al. (1985) filled certain classes of sensory neurons with fluorescein by simple immersion of animals in the dye. Mutants in five unc genes showed guidance defects in these neurons, with processes either growing erratically in abnormal locations, or stopping prematurely. Several mutants are also known in which the outgrowth of the touch neurons is defective (Chalfie and Sulston, 1983). Further studies have been undertaken using monoclonal antibodies (S. McIntire, S. Siddiqui and J. Culotti, unpublished) and by electron microscopereconstruction of mutants (J. White, unpublished).
The study of neural outgrowth undertaken here has concentrated on the ventral cord, and to a lesser extent the ganglia at either end (RVG and PAG). Figure 1.3 shows in schematic form all the neurons and nerve processes behind the RVG in a newly hatched L1 larva. The ventral cord contains the motor neurons that innervate body muscles as well as interneuron processes that run to and from the nerve ring. There are two groups of processes in the ventral cord, one on each side of the hypodermal ridge. They are very asymetrical. The right hand cord contains 25 to 30 processes, including the motor neuron processes and many pairs of interneurons which are bilaterally symmetric in the nerve ring, while the left hand cord contains only 3 or 4 processes. The other main longitudinal bundle is the dorsal cord, which contains motor neurons processes and just one interneuron, RID.
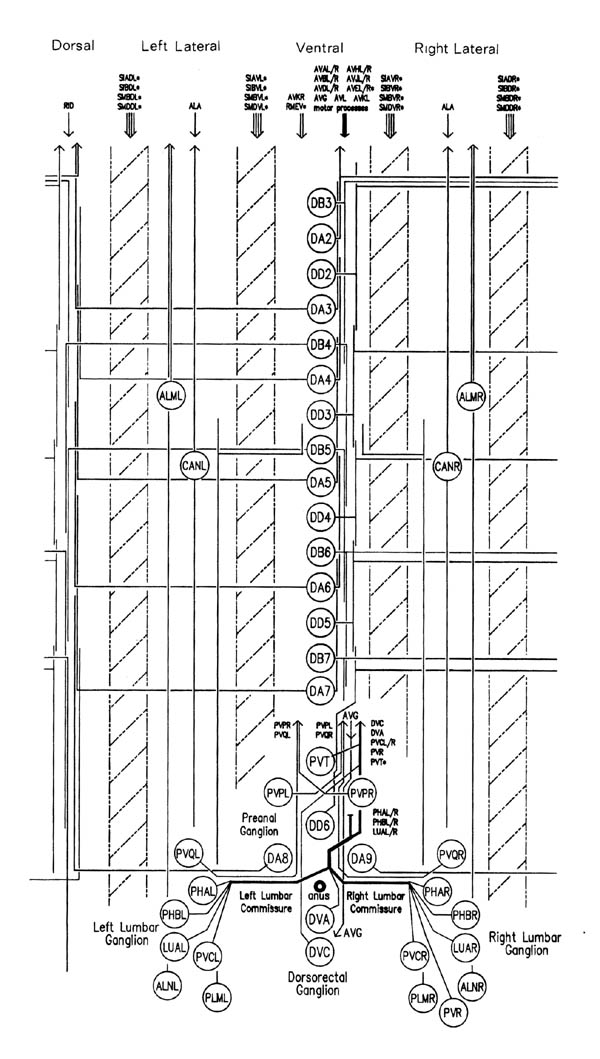 FIGURE 1.3. All the nerve processes and cell bodies behind the RVG. This diagram is a schematic cylindrical projection of the inner surface of the hypodermis and nervous system, obtained by conceptually cutting along the dorsal midline and unfolding flat. The dorsal cord is shown at the left hand edge, anterior is at the top, and posterior at the bottom. The positions of the four longitudinal muscle quadrants are shown by hatched regions. Nerve processes in C. elegans branch only rarely and reproducibly and all the branches in this region are shown. Processes entering the ventral, lateral or dorsal cords from the front are indicated at the top. Those with asterisk after the neuron's name only run part way back along the body. Posterior interneuron processes running forward along the ventral cord are indicated at the top. Those with an asterisk after the neuron's name only run part way back along the body. Posterior interneuron processes running forward along the ventral cord are indicated similarly at the front of the preanal ganglion. All the anterior axons in the ventral cord without an asterisk terminate in the preanal ganglion, except for that of AVG, which is shown ascending into the dorsorectal ganglion. The PHA and PHB neurons from the lumbar ganglia also have posteriorly directed processes that terminate in the phasmid sensilla. Note the different directionalities of outgrowth of the different ventral cord motor neuron classes.
FIGURE 1.3. All the nerve processes and cell bodies behind the RVG. This diagram is a schematic cylindrical projection of the inner surface of the hypodermis and nervous system, obtained by conceptually cutting along the dorsal midline and unfolding flat. The dorsal cord is shown at the left hand edge, anterior is at the top, and posterior at the bottom. The positions of the four longitudinal muscle quadrants are shown by hatched regions. Nerve processes in C. elegans branch only rarely and reproducibly and all the branches in this region are shown. Processes entering the ventral, lateral or dorsal cords from the front are indicated at the top. Those with asterisk after the neuron's name only run part way back along the body. Posterior interneuron processes running forward along the ventral cord are indicated at the top. Those with an asterisk after the neuron's name only run part way back along the body. Posterior interneuron processes running forward along the ventral cord are indicated similarly at the front of the preanal ganglion. All the anterior axons in the ventral cord without an asterisk terminate in the preanal ganglion, except for that of AVG, which is shown ascending into the dorsorectal ganglion. The PHA and PHB neurons from the lumbar ganglia also have posteriorly directed processes that terminate in the phasmid sensilla. Note the different directionalities of outgrowth of the different ventral cord motor neuron classes.
The ventral and dorsal cords contain the motor circuitry controlling body movement. There are three classes of motor neuron at the L1 stage, DA, DB and DD (five more classes are added during postembryonic development). In addition to having their cell body and a process in the ventral cord, all these motor neurons send a commissure round the body of the animal to the dorsal cord, where they have another process. Muscle arms from ventral muscles extend to the ventral cord, while those from dorsal muscles extend to the dorsal cord. Movement of the body is limited to the dorsal-ventral plane. The head has more freedom of movement, owing to more complex innervation of the muscles in the head directly from the nerve ring, but motion of the whole animal is caused by propogating dorsal-ventral waves along the body. DA and DB neurons both have their neuromuscular output in the dorsal cord, and receive input from (different) interneurons in the ventral cord. However they have different polarities: both ventral and dorsal DA processes grow forward, while DB processes grow backward. DD motor neurons receive input in the dorsal cord from the DA and DB neurons, by intercepting" their neuromuscular junctions, and have output in the ventral cord, which is thought to be inhibitory, ensuring relaxation of the ventral musculature while the dorsal musculature is contracted.
In addition to those in the ventral and dorsal cords there are a few neuronal cells and processes on the lateral hypodermal ridge and four small ganglia at the back of the animal (figure 1.3). The lateral neurons ALM and PLM are touch receptor classes (Chalfie and Sulston, 1980), while CAN and ALA are associated with the excretory canals, which run through the lateral ridges. In the front half of the animal there are four processes running back under each muscle quadrant from the nerve ring. These sublateral processes are possibly proprioceptive, involved in controlling head movement, since the neurons they belong to are closely associated with the head motor circuitry, SMBD and SMDD being motor neuron classes themselves. The preanal ganglion contains three interneuron cell bodies, DD6, DA8 and DA9. The lumbar ganglia on the sides at the back contain the cell bodies of the ALN and PLM neurons, which have lateral processes, and of the phasmid chemoreceptors PHA and PHB and the ventral cord interneurons PVQ, PVC, LUA and PVR, all of which send anterior processes down to the preanal ganglion and the ventral cord via the lumbar commissures. Finally there are two neurons in the dorsorectal ganglion on the top surface of the rectal epithelium behind the anus, DVA and DVC.
There are both practical and strategic reasons for choosing the ventral cord as the target for study. First, although the final anatomy of the nerve ring has been reconstructed, it is too complex a structure to be able to easily study its development. Its final structure is, however, discussed with respect to developmental considerations in the second part of this thesis. Second, the method of observation used has been reconstructed from electron micrographs, and it is relatively easy to reconstruct the ventral cord region from transverse sections, since processes are mostly longitudinal, any commissures containing only a few processes. Third, and perhaps most importantly, it is possible to at least some extent to examine functionality defects in ventral cord structure, which allows the combining of work on structure and function. A reasonable functional model of the ventral cord motor circuitry has been proposed, both by analogy to the results in Ascaris and as a result of ablation experiments in which components of the circuitry were removed (Chalfie et al., 1985). Movement is very easily observed, and a large number of uncoordinated mutants have been obtained that have various defects in movement (Brenner, 1974).
As mentioned previously, some of these mutants have been seen to have defects in nerve process morphology (Hedgecock et al., 1985 S. McIntire, J. White unpublished observations). Particular examples are that some or all circumferential commissures go astray in unc-5, unc-6 and unc-33 mutants, and the PHA and PHB processes get stuck at the bottom of the lumbar commissures in unc-33, unc-44, unc-51 and unc-76 mutants. These defects suggest that the affected genes may be involved in the processes of neural outgrowth that have been studied here. Genes defined in this way provide a possible link between the anatomical experiments and observations described here and the molecular mechanisms involved. The defects they induce are compared with the wild type development and the effects of cell ablations in Chapter 5.
Chapter 2: Materials and Methods
2.1 C. elegans neuronal nomenclature
2.2 Electron microscopy
2.3 Ablations
2.4 Reconstruction
2.5 Staging of reconstructed embryos
2.6 Identification of neurons
2.6.1 AVG
2.6.2 Ventral cord motor neurons
2.6.3 PAG cells
2.6.4 DRG cells
2.6.5 Lumbar ganglia cells
2.6.6 Lateral cells
2.6.7 RVG cells
C. elegans (var. Bristol, N2 strain) was propogated on lawns of E. coli grown on agar Petri plates, as described by Brenner (1974).
2.1 C. elegans neuronal nomenclature
The 302 neurons are divided into 118 classes on the basis of morphology and synaptic connectivity. Each neuron's name consists of two or three capital letters denoting the class and a suffix denoting which member of that class it is. The motor neurons in the nerve cord have two letter roots and a number as a suffix, so DA2 is the second member of the DA class (counting from the front). Interneurons have three letter roots and use the suffix letters L, R, D, and V to distinguish left, right, dorsal, and ventral members. Thus PVPL is the left member of the PVP class. Unique neurons, such as AVG, have no suffix. When referring to a class rather than one of its members merely the two or three letter route name is used.
2.2 Electron microscopy
Embryos were isolated by dissolving gravid adults with 1% hypochlorite, 0.5 M KOH for 5 mins, collecting the eggs through a 52 micron filter (Nitex) then rinsing the eggs three times in M9 buffer. The eggshells were digested with chitinase following the the procedure of Wolf et al. (1983), and the remaining viteline membrane was broken mechanically by pipetting the chitinased eggs through a drawn pasteur pipette. After removal of the eggshell the embryos were fixed in 1% OsO4, 0.8% KFe(CN)6 (0.1M cacodylate buffer, pH 6.0) for 45 minutes at room temperature. They were then rinsed in 0.05 M cacodylate buffer, pH 7.0, and treated for 15 mins with 0.2% tannic acid (Malinckrodt) in the same buffer. Finally they were rinsed in dH2O and straight embryos of approximately the right age were embedded, sectioned, and stained as in White et al. (1986). Adults were simply fixed for one hour in 1% OsO4 in 0.1 M NaPO4, pH 7.4, and cut in half before embedding to ensure proper infiltration. The sections were viewed on an AEI 6B electron microscope and photographs were taken every 2 to 3 sections (nominal section thickness, 50nm) at a magnification of 3 to 10 thousand. I am very grateful to J N Thomson, who did all the serial sectioning with uncanny consistency and reliability. J. Priess developed the fixation protocol used here.
2.3 Ablations
In order to remove specific cells from the developing nervous system the parent of the desired cell was ablated using a laser microbeam. To obtain embryos, gravid adults were cut open in a watch glass of distilled water (dH2O). About 30 embryos of approximately the right age were transferred to a 3% agar pad and grouped together. The surrounding agar was cut away to leave a 3mm x 10mm strip, and a cover slip was placed on top, held in place with dabs of hot vaseline on the corners. Extra water was added to prevent dessication. Under the slight pressure of the cover slip approximately half the embryos lie ventral side up, as desired, and the pattern of individual cell nuclei around the desired time can be reliably recognised using Nomarski optics (pattern shown in figure 2.1).
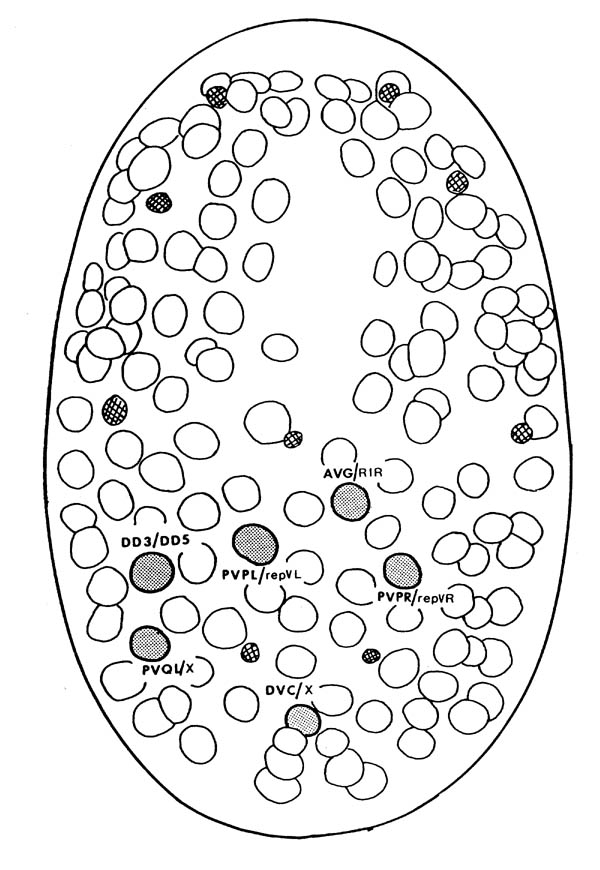 FIGURE 2.1. A line drawing of the position of the cell nuclei on the ventral surface of the embryo at 270 mins, the approximate time when the laser ablations were performed. Anterior is at the top of the page. Nuclei are clearly visible by Nomarski microscopy. Most neural precursor cells at this stage will divide one more time. Cells that were ablated are shaded and their normal daughters are shown. An X represents a cell that dies soon after birth. The smaller crosshatched cells are cells that die around the time of this picture; they are very distinctive and provide useful landmarks. (Adapted from Sulston et al., 1983).
FIGURE 2.1. A line drawing of the position of the cell nuclei on the ventral surface of the embryo at 270 mins, the approximate time when the laser ablations were performed. Anterior is at the top of the page. Nuclei are clearly visible by Nomarski microscopy. Most neural precursor cells at this stage will divide one more time. Cells that were ablated are shaded and their normal daughters are shown. An X represents a cell that dies soon after birth. The smaller crosshatched cells are cells that die around the time of this picture; they are very distinctive and provide useful landmarks. (Adapted from Sulston et al., 1983).
Ablations were executed with a pulsed laser (PRA LA1000/LN102 used with Courmarin 450 dye), whose beam is focussed down the microscope objective as in Sulston and white (1980). The chosen cell was killed with repeated low energy laser pulses (20-100 hits). After 15-20 mins the dead cell shrinks into a condensed refractile ball. If it is on the ventral surface, as with all but one (DVC) of this set of experiments, then it is excluded from the embryo when the hypodermis, which starts as a patch on the dorsal side, closes over about 45 mins after the ablation (figure 2.2).
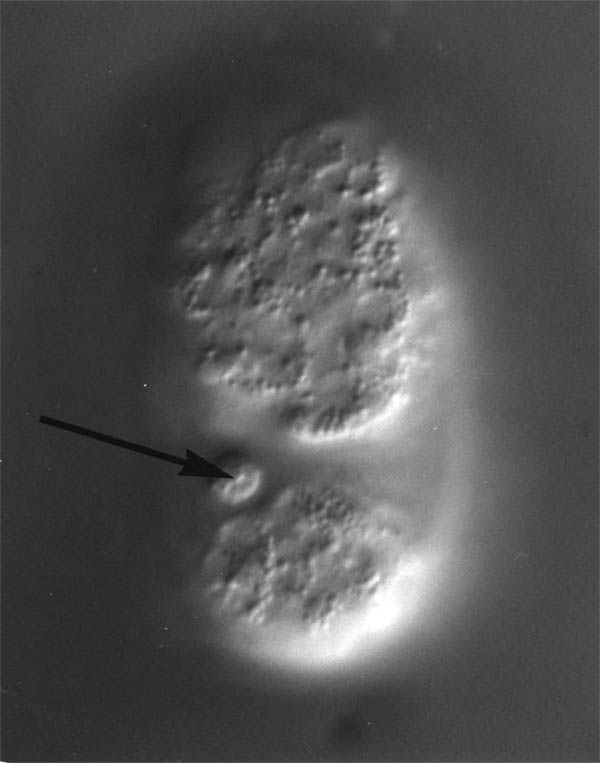 FIGURE 2.2. After ventral surface cells are killed the remains are excluded from the embryo when the ventral hypodermis seals up at around 320 mins. Here the ablated parent of PVPL is shown at approximately 350 mins (arrowed). The total length of the egg is 60 microns.
FIGURE 2.2. After ventral surface cells are killed the remains are excluded from the embryo when the ventral hypodermis seals up at around 320 mins. Here the ablated parent of PVPL is shown at approximately 350 mins (arrowed). The total length of the egg is 60 microns.
After monitoring exclusion of the dead cell in the experimental embryos, they were transferred either to petri dishes with bacteria if they were to hatch, or, if they were to be fixed as older embryos, to an 8 well multi test well slides (Flow Labs) subbed with 0.1% polylysine. The fixation protocol as above, all the fixation steps being carried out with the embryos inside their eggshells attached to the test well slides. In order to allow access of fixatives etc. to the embryos, the laser wa sued to make a small hole in the eggshell in the presence of the first (OsO4) fixative at the chosen stage of development. Fixed embryos were dislodged from their slides and embedded and sectioned as above.
2.4 Reconstruction
Prints were made from each negative and the reconstruction was carried out directly from the prints by writing a label inside each profile with a drafting pen and following the labels from one photograph to the next. In many cases nerve cells and processes were immediately identifiable, but when this was not so an arbitrary label was used and the cell was identified later if possible. The criteria used for neuron identification are given below. Aside from the problem of identification of a correctly reconstructed cell there may be problems in forming a continuous reconstruction itself. Usually these problems are generated either by a number of consecutive sections being unphotographable because of grid bars or dirt on the grid covering the sections, or by the neuropil being cut tangentially to some nerve processes so that the membrane boundaries become indistinct. In all the cases considered here these problems were satisfactorily resolved, when necessary by checking internal consistency (e.g. a process with two attached cell bodies is no good, nor is an unattached process) or consistency with the equivalent cells in other reconstructions. One embryonic AVG ablation reconstruction was abandoned because processes could not be definitely linked across a break. Generally these difficulties are less severe in embryonic than in adult reconstructions, since there are many fewer processes in each bundle and the processes have smoother trajectories; they are not so tightly constrained by other tissues, particularly since the muscle is till not fully developed.
Altogether 19 reconstructions of varying regions of different embryos were undertaken, using around 3000 photographs.
2.5 Staging of reconstructed embryos
None of the reconstructions described in this dissertation came from timed embryos. Stages were assigned by placing them in a developmental sequence and comparing them with short serial reconstructions from less ideal embryos of known age at fixation, and with previously known developmental events that were detectable in the reconstructions (e.g. cell divisions and movements). The timed embryos were obtained by cutting open gravid adults and selecting embryos at the two cell stage. These were incubated at 25°C and then fixed by the same method as the ablation experimental embryos (above). Development times at 25°C were converted to times at 20°C from standard growth curves (Schierenberg, 1978).
2.6 Identification of neurons
There are several factors that make cell and process identification from electron microscope reconstructions relatively straightforward in C. elegans embryos. To begin with, there is simply not very much there. Figure 3.7 show typical ventral cord and preanal ganglion sections. What cells there are are sufficiently different from one another to be easily and reproducibly distinguishable. The positions and identities of all the cell bodies are known throughout embryonic development from the remarkable work of Sulston et al. (1983) obtained by light microscopy with Nomarski optics. All the cells under consideration here have an invariant lineage, and their relative cell body positions are extremely reproducible. Second, the nerve process morphologies are simple enough to be fully traceable in the reconstructions. They are also highly reproducible and all their adult forms are known from the equally enclylopaedic work of White et al. (1986). Figure 1.3 shows the approximate positions of all the neurons behind the RVG (see also Sulston et al. 1983 for camera lucida drawings at different stages). In general all cell bodies and processes were identified in all reconstructions. I give below the specific criteria used to identify the various cells, followed by a discussion of the remaining cases where complete identification was not possible.
2.6.1 AVG
The ventral cord process was followed back to a cell body in the RVG in the A and B wild type reconstructions. AVG is the only neuron in the RVG to send a process back along the whole length of the ventral cord, and the position of the cell body was as expected in each case. In other reconstructions AVG was identified by the fact that it was the only continuous process in the ventral cord (if the series was early enough) or because it is the only process to grow into the DRG (DVA and DVC have cell bodies in the DRG and grow down out of it). Ablation of AB.prpapppa, the parent of AVG, removed the ventral cord process that had been identified as AVG.
2.6.2 Ventral cord motor neurons
These were identifiable by cell body order and the direction of outgrowth of processes and commissures, which were known to be invariant from larval and adult reconstructions. In all cases unique identifications could be made which were entirely compatible to the known data (except in the AVG parent ablations commissure direction was altered though the order of cell classes remained as normal). In the early series, before commissures grow out, the cell bodies overlap and there is a vertical order, with DA cells overlapping dorsally to DD cells, which in turn are dorsal to DB cells (consistent with Normarski observations of Sulston et al., 1983).
2.6.3 PAG cells
The relative positions of PAG cell bodies are shown in figure 1.3. The only variability that was found in reconstructions was that the body of DD6 was sometimes more anterior, underneath PVPL, PVPR and PVT. The following diagnostic criteria confirmed assignments: PVT never sent out a process in any embryonic reconstruction and always was the most anterior ventral ectodermal cell to contact the rectal epithelium (repVL and repVR). PVPL and PVPR have a unique process morphology in the PAG since their processes cross over when they leave their cell bodies, and then grow forward along opposite sides of the cord. DD6 has a standard DD type process; also the PVQL process and, to a lesser extent, PVQR and DVC processes tend to flatten out on the surface of DD6. DA8 and DA9 are the only cells to send processes up the lumbar commissures (left and right respectively). Ablation of AB.prppppaa or AB.plppppaa, the parents of PVPR and PVPL respectively, resulted in the correct PVPL/PVPR cell being missing and an accommodation in position by the other cells in the PAG (Chapter 4).
Note that I have named the PVP cells by the position of their cell bodies and lineage, in accordance with the general practice for C. elegans neuronal nomenclature and with Sulston et al. (1983). The ablations confirm that the cells do not exchange positions after being born. Since their processes cross over this means that the PVPR process is on the left. This is reversed from the nomenclature of White et al. (1986), in which PVPR has its process on the right. The reason for this inconsistency is that the PVP cells are squashed into a line in the adult and the crossover is not apparent. The same holds for the RIF, RIG and SABV cell pairs in the RVG, whose processes also cross over, and which I have also named in accordance with Sulston et al. (1983), rather than White et al. (1986).
2.6.4 DRG cells
DVA and DVC are the only embryonic cells in the DRG. Whenever their processes were seen, except in the anterior D reconstruction, they were followed back to the PAG. In cases where they were not followed back to their cell bodies they were distinguishable because of very different behaviour in the PAG (see below), and because the DVA process descends into the PAG around the right side of the rectum, whilst the DVC process descends around the left side.
2.6.5 Lumbar ganglia cells
The relative positions of cells in the lumbar ganglia are shown in figure 1.3. This region was only reconstructed once, in the wild type C reconstruction. In other cases the PVQ processes in the ventral cord were identified by (I) their characteristic behaviour in the PAG, and strong association with PVP processes (figure 3.7), (ii) the fact that they were by far the most advanced processes coming out of the lumbar commissures. PVQL is the only lumbar commissure process that runs on the left side of the ventral cord (White et al., 1986). The ablation of AB.plapppaa, the parent of PVQL, removed the PVQL ventral cord process (Chapter 4). The process of other lumbar ganglia cells were only separately identified in the C reconstruction. In other cases they were identified as a group.
2.6.6 Lateral cells
The few neurons with cell bodies lying on the lateral hypodermis (figure 1.3) are well spaced out and can easily be identified on the basis of cell position.
2.6.7 RVG cells
The RVG was only reconstructed in the A and B wild type reconstructions. AVG was identifiable by its posterior process. The three bilateral sets of cells (RIF, RIG, SABV) could be paired off according to position, size and process growth. Other cell identifications were made on the basis of position, and are not completely definite. However the only cells that I discuss below are AVG, and the RIF and SABV neurons and their identifications are certain.
The only cases apart from the lumbar commissure processes and the RVG in which definite identifications were not made are in the anterior D reconstruction. Here the majority of interneurons cannot be individually identified. On the left side of the cord only two processes are present at the posterior end of the reconstruction, so they must be PVQL and PVPR, since they grow forward together from the back. There is also one anterior process running part way back. This could either be AVKR or RMEV. On the right side there are 4 processes present at the front of the reconstruction that terminate at some point before the back. These are presumably interneurons with cell bodies around the ring, but to identify them individually would require reconstructing the entire nerve ring region. There are also 7 processes running through the entire reconstruction, which probably include PVPL and PVQR since the left hand versions of these have grown right through the reconstruction. It is not possible to identify the others.
Chapter 3: The Pattern and Outgrowth in Normal Embryos
3.1 Morphology of growth cones
3.2 The attachment substrate for growth cones
3.3 AVG pioneers the ventral cord
3.4 Motor neurons
3.5 Later ventral cord interneurons
3.6 Decussation in the preanal and retrovesicular ganglia
3.7 Growth cone insertions into other cells
The organisation of processes in the ventral nervous system is established during a short period of little more than an hour, at the same time as the animal is elongating in the eggshell from a stubby "tadpole" to a worm. Electron microscope reconstructions of varying lengths were made from a series of four embryos at different developmental stages during this period (figure 3.1). Figure 3.4 shows a schematic picture of the state of the ventral nervous system in each of the reconstructions, which will be referred to by the letters A to D. During the period covered by these reconstructions the embryo increases by a factor of about two in length, being about one and a half fold in the egg (100 microns) at the time of the A reconstruction, and three and a half fold (220 microns) in the D reconstruction. At the beginning of the period under consideration here the nerve ring contains the majority of the final number of processes. Uncoordinated muscle activity has already started before the time of the A reconstruction (the onset of twitching is at about 430 mins). Movement becomes more organised around the stage of the final, D reconstruction, although since the embryo is restricted inside the egg shell it is not possible to assess fully the degree of coordination.
3.1 Morphology of growth cones
Growth cones are generally extended flattened lamellar structures that also have long thin filopodial extensions. In C. elegans the most extensive growth cones are seen on the growing tips of the motor neuron commissures. Typically they are a flattened sheet a few tenths of a micron thick and of variable shape and size in the plane of the sheet (figures 3.2, 3.5). The absence of normal looking filopodia may be due to the small scale (2-5 microns across); a vertebrate tissue culture growth cone could extend right round the C. elegans embryo. However stubby finger-like extensions are seen in many cases, and these may perform an equivalent function. Figure 3.2 shows a three dimensional reconstruction of the complete cell DB4 from the B reconstruction, in which the thin sheet-like nature of the growth cone can be clearly seen.
Extended growth cones like those seen on commissures were not seen on processes growing along the ventral or dorsal cords, although some tips do have swollen or spread out endings (e.g. PVCL in figure 3.7). This corresponds to observations made in other organisms that process growing along pre-existing nerve bundles do not have such extended growth cones as those growing over virgin territory (Lopresti et al., 1973).
The quality of the cytoplasmic fixation in the embryos used for reconstruction was poor, since primary fixation is with OsO4 followed by tannic acid, which fixes membranes well but leaves little cytoplasmic structure. Therefore neither actin microfiliaments nor microtubules are preserved. However in some cases it is possible to see vesicles in growth cones, as for example in a commissural growth cone in the C reconstruction (figure 1.1). Studies by de Cino (1981) have indicated that transmitter is sometimes released by growth cones. An alternative explanation for the vesicles is simply that they may be a source of new membrane for insertion at the leading edge of the growth cone.
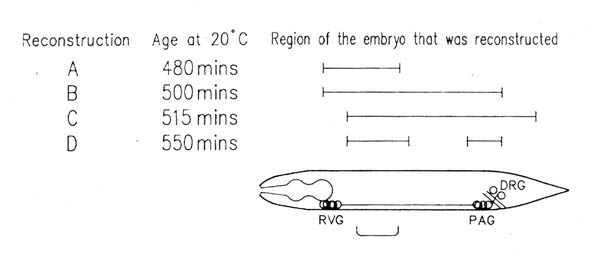 FIGURE 3.1. This shows the approximate ages of the embryos used in this study from which long series were reconstructed completely, and the parts of them that were reconstructed. Ages were determined as described in Chapter 2.5. The bracket below the embryo indicates the part of the ventral cord shown in figure 3.4.
FIGURE 3.1. This shows the approximate ages of the embryos used in this study from which long series were reconstructed completely, and the parts of them that were reconstructed. Ages were determined as described in Chapter 2.5. The bracket below the embryo indicates the part of the ventral cord shown in figure 3.4.
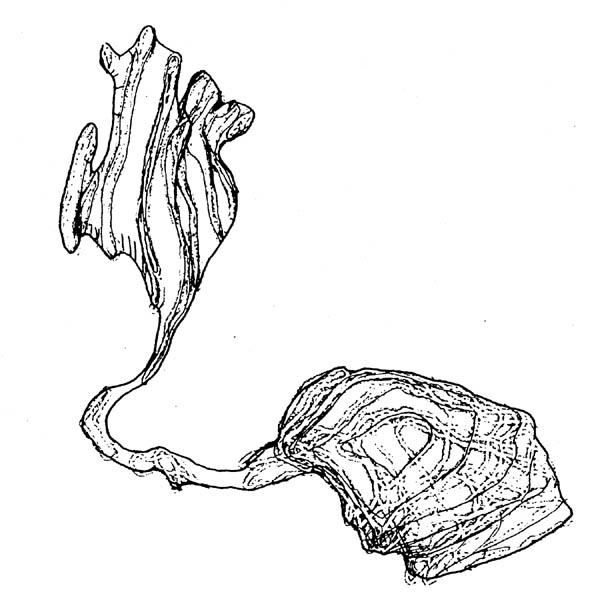 FIGURE 3.2. A three-dimensional reconstruction of the motor neuron DB4 from the B series. The cell body is on the right. Out of this extends a growing commissure, terminating in the flattened extended structure at the left, which is the growth cone. This diagram was made with the aid of a 3-D reconstruction program written by J.G. White. The growth cone is approximately 5 microns across.
FIGURE 3.2. A three-dimensional reconstruction of the motor neuron DB4 from the B series. The cell body is on the right. Out of this extends a growing commissure, terminating in the flattened extended structure at the left, which is the growth cone. This diagram was made with the aid of a 3-D reconstruction program written by J.G. White. The growth cone is approximately 5 microns across.
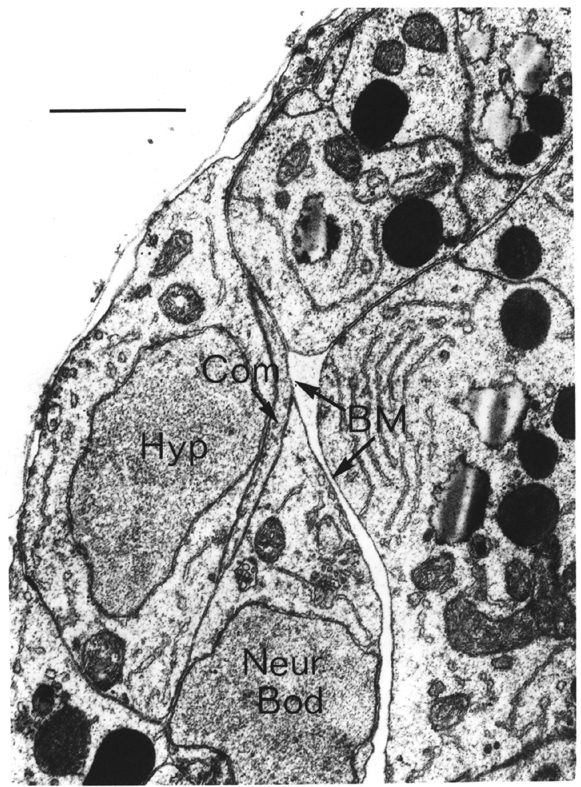 FIGURE 3.3. The growing DB5 commissure (Com) is forced to choose whether to pass the lateral neuron body of CANL (Neur Bod) on the side of the hypodermis (Hyp) or on the side of the basement membrane (BM). It passes on the hypodermal side, as do all motor neuron commissures in similar situations. This suggests that commissural growth cones attach to and move over cell surfaces rather than basement membrane. From the C reconstruction. Scale is 1 micron.
FIGURE 3.3. The growing DB5 commissure (Com) is forced to choose whether to pass the lateral neuron body of CANL (Neur Bod) on the side of the hypodermis (Hyp) or on the side of the basement membrane (BM). It passes on the hypodermal side, as do all motor neuron commissures in similar situations. This suggests that commissural growth cones attach to and move over cell surfaces rather than basement membrane. From the C reconstruction. Scale is 1 micron.
3.2 The attachment substrate for growth cones
Growth cones have been seen in vitro to extend very well over artificial substrates made of basement membrane components, such as fibronectin and laminin (Baron van Evercooren et al., 1982). This has led to the suggestion that basement membrane may provide a favoured substrate for growth cones to grow over. It is possible in at least one case in C. elegans to determine the substrate on which the growth cone moves. The motor neuron commissures grow out sandwiched between hypodermal cells and the basement membrane. There are several lateral neuronal cells that also lie between the hypodermis and the basement membrane, in the way of the growing commissures. Whenever a commissural growth cones reaches a lateral cell body it leaves the basement membrane and passes between the hypodermis and the lateral neuron (figure 3.3). There has never been observed an exception to this rule. Thus it seems that the growth substrate for these commissures is the surface of hypodermal cells, not the basement membrane.
A couple of similar results are provided by ablation experiments (Chapter 4) in which in one case DD5 moves from the right side of the cord to the left (after removing AVG), and in another case PVQL moves from the left to the right (after removing PVPR). In each case the process changing sides passes under a motor neuron cell body, rather than over it, again maintaining contact with the hypodermis rather than the basement membrane. There are many other examples where processes grow between cell bodies and other processes, well removed from the ectodermal basement membrane. During later development several posteembryonic processes grow the length of the ventral cord in the middle of the main bundle of embryonic processes. The embryonic reconstructions presented here show processes from the lumbar ganglia growing forward through the middle of the cluster of cell bodies in the preanal ganglion. In general wherever there is evidence on the subject of neuronal growth cone guidance in C. elegans it suggests that the substrate for growth is the surface of other cells, rather than a basement membrane. However this does not rule out the possibility that the basement membrane is important in certain cases.
3.3 AVG pioneers the ventral cord
The first nerve process to grow along the ventral cord belongs to the interneuron AVG. AVG has its cell body in the retro-vesicular ganglion at the front of the ventral cord; it is an unpaired cell, and is the most posterior interneuron in the front of the animal to send a process back along the cord. The cell body and process were identified in both the A and and B reconstructions. By the A reconstruction the process has already grown back along the cord. At this stage the DD ventral cord processes have also grown out on the right side of the cord (figure 3.4). However inspection of a younger embryo revealed that there was a single continual process in the ventral cord at a stage at which DD processes had not grown out (not shown). In the B reconstruction the AVG process grows the full length of the cord and up out of the pre-anal ganglion into the dorso-rectal ganglion, where it stops by the DVC cell body. It reaches no further than the DRG in all the latter embryonic reconstructions (B to D), although in the adult it is seen to extend right back into the tail spike (White et al., 1986). Therefore there must be a second period of extension postembryonically or during late embryogenesis.
3.4 Motor neurons
The next event after the appearance of AVG is the growth of processes from the DD motor neurons forward alongside the AVG process on the right side of the ventral cord. In the A reconstruction these extend until they nearly touch the next DD cell body (figure 3.4). In the adult, adjacent DD neurons overlap for a short stretch and are linked by gap junctions, but in all the embryonic reconstructions there are small spaces between them of the order of a micron in length (figures 3.4, 3.5). It is of course possible that contact has been made and the processes subsequently withdrawn. Another possible correlate of DD extension in the ventral cord, discussed further later, is that all the DD ends of DD processes are by DB cell bodies. Ventral cord processes from the DA and DB motor neurons do not grow out until later, after the commissures and dorsal cord processes are made.
The first signs of commissure outgrowth can also be seen in the A reconstruction. All the motor neurons have lamellar extensions poking laterally under the ventral musculature at the site where their commissural growth cones will leave the ventral cord (figure 3.4). These nascent growth cones leave from the DA and DB cell bodies, and from near the anterior end of DD axons. Outgrowth of commissures from the motor neurons of all classes is synchronous; in the B series, only about 20 minutes older than the A series, they have all reached the lateral hypodermis, and in the C series they are just about to reach the dorsal hypodermal ridge (figure 3.4). RID, the only process to grow along the full length of the dorsal cord, is not present at this time, and the commissures apparently turn of their own accord, DA ones anteriorly, DB posteriorly, and DD in both directions, and link up to form the dorsal cord. Although they are reproducible, there is no regular anterior/posterior order to the positions of the commissures from the different classes of cells (figures 1.3, 3.5), so the direction in which they turn cannot be simply determined on the basis of the classes of neighbouring processes (e.g. DA's and DB's towards each other). Since the dorsal hypodermis is syncytial and does not contain apparent landmarks it seems that the direction must be intrinsically determined. In addition, if we assume that the direction is determined in the same fashion for all the members of a class, then it must be specified in terms of the anterior and posterior of the animal, rather than whether to turn left or right, since some of the DB axons go round the left hand side of the body and turn right when they reach the dorsal midline, while others go round the right side and turn left.
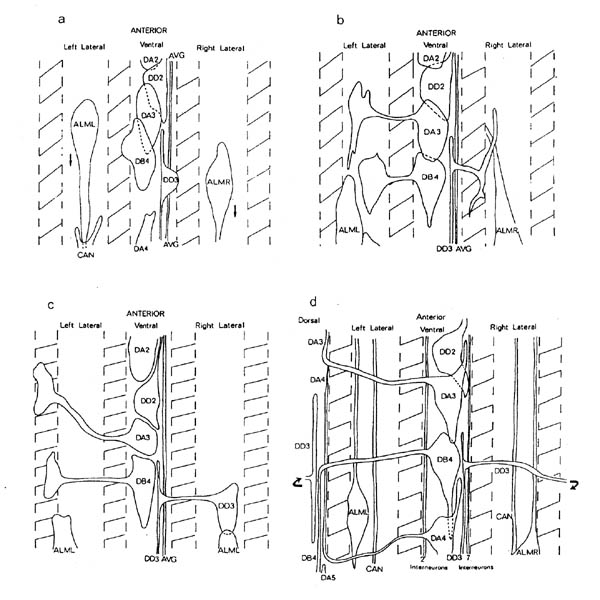 FIGURE 3.4. Schematic diagrams of the part of the body indicated in figure 3.1 from the A, B, C and D reconstructions (1), b), c) and d) respectively). The diagrams are cylindrical projections in the same basic form as figure 3.1, with the positions of the muscle bands being shown as hatched areas. The DA3, DB4 and DD2 cell bodies and the DA3, DB4 and DD3 commissures are shown in each case. Initially only the AVG and DD processes in the ventral cord are present (a). Then the commissures grow out simultaneously from all three motor neuron classes (b, c), followed eventually by the ventral cord processes of DA and DB motor neurons and other ventral cord interneurons (d). ALM and CAN are lateral neurons, which migrate back from the front and then send processes forward.
FIGURE 3.4. Schematic diagrams of the part of the body indicated in figure 3.1 from the A, B, C and D reconstructions (1), b), c) and d) respectively). The diagrams are cylindrical projections in the same basic form as figure 3.1, with the positions of the muscle bands being shown as hatched areas. The DA3, DB4 and DD2 cell bodies and the DA3, DB4 and DD3 commissures are shown in each case. Initially only the AVG and DD processes in the ventral cord are present (a). Then the commissures grow out simultaneously from all three motor neuron classes (b, c), followed eventually by the ventral cord processes of DA and DB motor neurons and other ventral cord interneurons (d). ALM and CAN are lateral neurons, which migrate back from the front and then send processes forward.
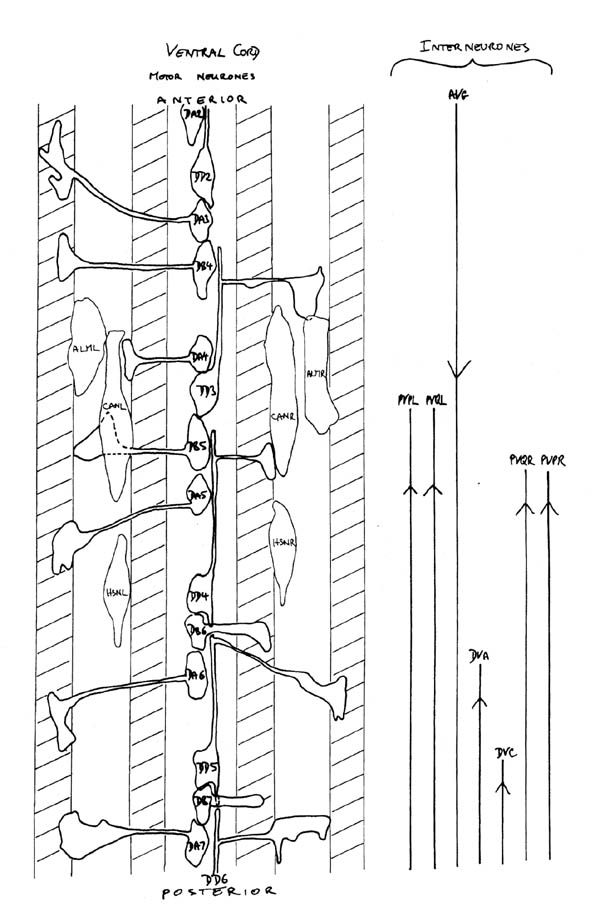 FIGURE 3.5. A schematic diagram of the entire ventral cord region from the C reconstruction. The motor neurons and lateral neurons are shown on the left in the same form as in figure 3.4. (HSN are postembryonic neurons that grow out processes in the L4 larva to innervate vulval muscles). The positions reached by all the interneurons that have grown substantially into the ventral cord are shown on the right. In addition to the PVT, PVCL and PVCL processes have also grown just past the front of the preanal ganglion in this reconstruction.
FIGURE 3.5. A schematic diagram of the entire ventral cord region from the C reconstruction. The motor neurons and lateral neurons are shown on the left in the same form as in figure 3.4. (HSN are postembryonic neurons that grow out processes in the L4 larva to innervate vulval muscles). The positions reached by all the interneurons that have grown substantially into the ventral cord are shown on the right. In addition to the PVT, PVCL and PVCL processes have also grown just past the front of the preanal ganglion in this reconstruction.
There appears to be a possible correlation between the position of DA commissures and the location of hypodermal cell boundaries. The embryonic ventral hypodermis consists of 6 left/right pairs of cells, known as P cells, which are joined at front and back to the main body hypodermal syncytium, called hyp7. DA3 to DA7 lie on the boundaries between adjacent P cells, and they send solitary commissures to the left directly from their cell bodies out along the P/P cell boundary. In contrast DA2, which is on the boundary between the most anterior P cells and hyp7, sends its commissure forward and out to the right together with those of DB3 and DD2. Similarly DA1, DA8 and DA9, none of which are near a P/P cell boundary, send their commissures together with processes from other cells (DB2 and DD1 in the case of DA1, the lumbar commissures for DA8 and DA9).
There are no corresponding visible cues for DB commissure guidance, and only weak ones for DD commissures. One possibility is that DB cells are involved; DD commissures all leave the cord from approximately opposite DB cells (figure 3.5, note especially DD3, whose commissure exit point is quite a long way behind the DD2 cell body, but opposite DB4). It is hard to tell whether a DD commissure is created by diversion of the growth cone that generated the ventral cord process, or by a genuine branching. The commissure always comes from near the anterior tip of the ventral cord process, the extension of which is essentially complete when the commissure starts growing, suggesting that only a single growth cone is used. However there is a definite T junction in the final structure, and the DD process can make branches, since one is certainly made when the commissure reaches the dorsal cord (figure 1.3). The side of the animal that the DD commissures go round is easier to explain. They all go round the right side of the animal, which is consistent with the position on the right side of the cord of their ventral cord processes, from which the commissures diverge or branch.
It is only after the dorsal cord processes have extended for some distance that we begin to see growth of the ventral cord dendrites from DA and DB neurons, (D reconstruction, figure 3.4). This coincides with the growth back along the cord of some of the ring interneurons, possibly including the motor circuitry interneurons that innervate the DA and DB ventral cord processes. However there are no visible synaptic connections between the interneurons and the growing DA and DB dendrites.
The dorsal cord, in the other hand, does show signs of synaptic activity in the D reconstruction. The DA and DB neurons are already making small, but clear, neuromuscular junctions from their dorsal cord axons (figure 3.6). As in the final adult version these involve a joint synapse onto muscle and a DD process. No corresponding DD neuromuscular junctions in the ventral cord have been seen in this reconstruction. The dorsal cord processes have not reached their final length in the D reconstruction, and in fact are at a rather interesting stage: each DA axon stops where the next one arrives at the dorsal cord and turns. Later the axons overlap considerably, but the regions of neuromuscular output do not; instead they correspond closely to the regions where the axons are present at this stage. It is possible to speculate that there is a pause in axon extension while the zones of neuromuscular activity are being set up, but more data would be required to provide respectable evidence!
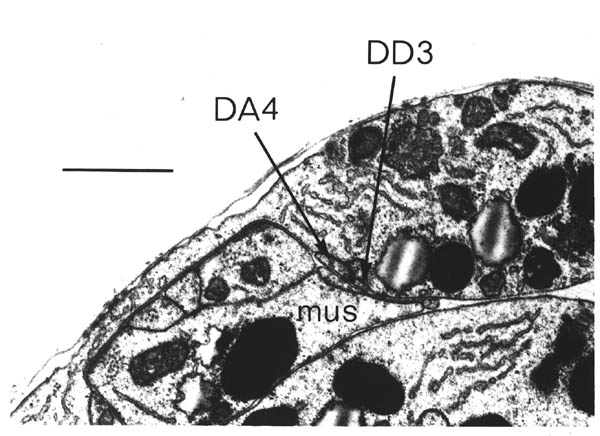 FIGURE 3.6. A neuromuscular junction from the DD reconstruction. Although small this shows all the characteristics of normal neuromuscular junctions in the developed nervous system. The dorsal cord process of the motor neuron DA4 is synapsing jointly onto muscle (mus) and the DD3 process. There is also a DB4 process present in this section. Scale bar is 1 micron.
FIGURE 3.6. A neuromuscular junction from the DD reconstruction. Although small this shows all the characteristics of normal neuromuscular junctions in the developed nervous system. The dorsal cord process of the motor neuron DA4 is synapsing jointly onto muscle (mus) and the DD3 process. There is also a DB4 process present in this section. Scale bar is 1 micron.
3.5 Later ventral cord interneurons
While the motor neuron commissures are growing out, a set of interneurons are growing forward along the ventral cord from the pre-anal ganglion (PAG) at the back (figure 3.5). The most advanced of these are two pairs of processes, PVPR and PVQL on the left side of the ventral cord and PVPL and PVQR on the right side. These are followed by DVA and DVC, which are both unpaired neurons that run on the right. Figure 3.7 shows a cross section of the posterior cord from the C reconstruction, after these posterior neurons have reached the front of the cord, that we see other anterior interneurons growing back along the cord (figure 3.4).
PVP and PVQ: The PVQ neurons are the most anterior cells in the lumbar ganglia (PVQL in the left lumbar ganglion, PVQR in the right, figure 1.3); their processes descend the lumbar commissures and then run forward through the PAG and along the cord. PVPR and PVPL have cell bodies in the PAG, where they are the only bilateral pair of interneurons. Their processes cross over when they leave the bodies, joining up with the PVQ process on the opposite side, and then run forward along the cord. The structure of PVP decussation is discussed below together with that of three pairs of neurons in the retrovesicular ganglion.
PVQL and PVPR pioneer the left hand ventral cord; this appears to be a joint action, since their anterior tips are never more than a few tenths of a micron apart in any of the reconstructions (e.g. figure 3.5). The tips of the PVQR and PVPL are similarly close to each other, but there is no such tight relationship between the left hand pair and the right hand pair (figure 3.5). The fact that the left hand pair are often more advanced than the right hand pair suggests that the prior presence of AVG and DD processes on the right side of the cord has little effect on PVPL and PVQR outgrowth. The two processes in each pair are tightly associated all the way along the cord back to the point where the PVP processes cross over in the PAG. This association is also seen in the adult reconstructions of the ventral cord; the processes diverge only when they reach the nerve ring (White et al., 1986, unpublished data). Together these observations suggest that PVP and PVQ growth in the ventral cord might be cooperative, and a number of ablation experiments were performed to test this hypothesis (Chapter 4). In general the PVP process is on top of the PVQ process, i.e. there is a ventral to dorsal order of: hypodermis, PVQ, PVP, basement membrane (figure 3.7).
DVA and DVC: DVA and DVC are the two embryonic neurons with cell bodies in the dorso-rectal ganglion, above the rectum (figure 1.3). They both grow forward along the right hand side of the cord behind PVPL and PVQR, but their tips are not close together like those of a PVP/PVQ pair. DVA grows down around the right side of the rectum back along the track of AVG, and in all cases keeps to the outside of the main right hand bundle of processes in the ventral part of the PAG (figure 3.7). DVC, on the other hand, grows down the left side of the rectum and crosses from dorsal left to ventral right, in the same place that two PVP processes cross over (figure 3.8). Neither DVA nor DVC appear to be particularly tightly associated with any other process in the ventral cord. In the adult cord DVA is always at the ventral right hand extremity of the main right hand bundle, whilst DVC runs in the middle of the bundle, in association with DVB, which is the postembryonic dorso-rectal ganglion neuron (White et al., 1986).
Other lumbar commissure processes: In addition to the DA8, DA9 and PVQ processes the lumbar commissures contain processes descending into the pre-anal ganglion from the following lumbar ganglion cells: PHAL/R, PHBL/R, LUAL/R, PVCL/R and PVR. Of these the PVC cells and PVR eventually grow the full length of the cord; the others stop at the front of the pre-anal ganglion. There is a very characteristic pattern at the back of the pre-anal ganglion where the lumbar processes from the two sides meet, which has been seen whenever the region has been reconstructed (figure 3.9). The processes from each side meet slightly to the right of the midline and "zip up", each contacting its contralateral analogue. The exceptions to this rule are the PVQ processes, which are at the top of the row one each side but stay at opposite corners of the structure and do not make contact. The dorsal to ventral order of this structure is PVQ, PHA, PHB, PVC, with the unpaired process of PVR wrapping around the ventral side of the whole group. This suggests that the contralateral pairs other than the PVQ's have a strong affinity for each other, and it is probably significant that the PVQ's are the only lumbar processes to run up the cord with one process on each side of the cord, as opposed to the more normal pattern of both processes being on the right.
Anterior interneurons: Anterior interneurons other than the AVG are only seen growing back along the cord in the D reconstruction. As described in the section on cell identification in Chapter 2 it is not possible to identify these processes. However it is clear that there are at least PVQR and PVQL on the left side. There is one process growing part way back along the cord on the left, which may be RMEV or AVKR, and there are 11 interneurons on the right, some of which probably come from the back (e.g. PVPL and PVQR), but others of which are from the front; in particular 4 from the front stop within the anterior D reconstruction. The anterior interneurons seen here may include (some of) the major interneurons that innervate the ventral cord motor neurons.
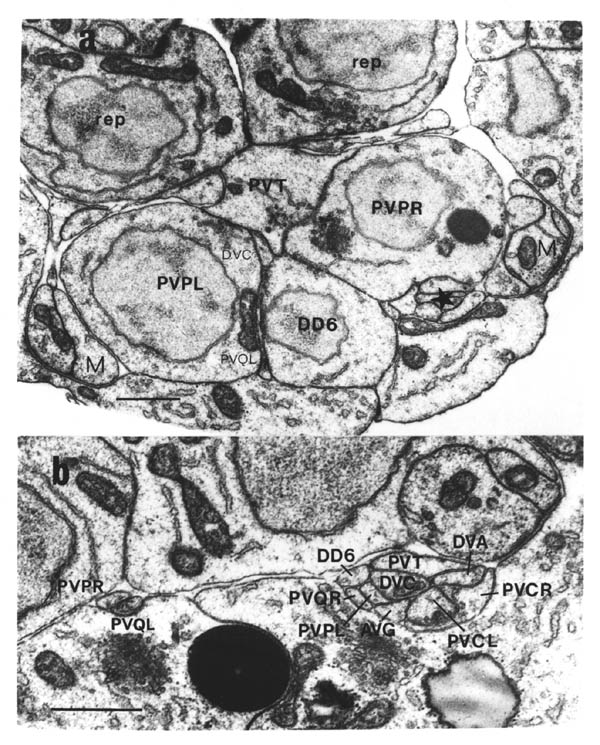 FIGURE 3.7. Typical transverse sections through (a) the preanal ganglion and (b) the back of the ventral cord (from the C reconstruction). In (a) the star indicates the main group of processes that will become the right hand ventral cord. The other cells and processes are individually labelled. This section comes from just posterior to the point where the PVP processes and DVC will cross over (shown in figure 3.8). In (b) the independent growth of PVPR and PVQL along the left hand cord is clear. This section comes from very close the the preanal ganglion, and the PVCL/R and PVT processes are near their front tips, and are a little swollen, particularly PVCR, which is showing signs of wrapping around other processes. Scale bar is 1 micron in each case.
FIGURE 3.7. Typical transverse sections through (a) the preanal ganglion and (b) the back of the ventral cord (from the C reconstruction). In (a) the star indicates the main group of processes that will become the right hand ventral cord. The other cells and processes are individually labelled. This section comes from just posterior to the point where the PVP processes and DVC will cross over (shown in figure 3.8). In (b) the independent growth of PVPR and PVQL along the left hand cord is clear. This section comes from very close the the preanal ganglion, and the PVCL/R and PVT processes are near their front tips, and are a little swollen, particularly PVCR, which is showing signs of wrapping around other processes. Scale bar is 1 micron in each case.
3.6 Decussation in the preanal and retrovesicular ganglia
The PVP processes cross over in the pre-anal ganglion where they leave their cell bodies and then grow forward on the opposite side of the cord. This crossing over is at the back of PVT and above DD6, at the same place that the DVC process crosses from left to right. PVQL, DVC and, to a lesser extent, PVQR flatten out on the surface of DD6; DVC is always between the PVP crossover and DD6 (figure 3.8). The PVP crossover was observed in the adult reconstruction (White et al, 1986), but it is much less clear there since there are extra cells in the adult preanal ganglion (6 postembryonic motor neurons) and the arrangement of cells and processes is much less well organised. This loss of symmetry and order is already visible in the difference between the B and D reconstructions. In the B reconstruction the cells in the left/right pairs (PVPL/R and DA8/DA9) are nearly opposite one another, while in the D reconstruction there is a definite tilt to each pair and the whole preanal ganglion is becoming more linear. This is probably caused by a lateral constriction due to elongation of the embryo and an increase in the amount of space taken up by the muscle cells as they mature. In the adult the symmetry and order present in the early embryo when processes first grow out is almost entirely lost.
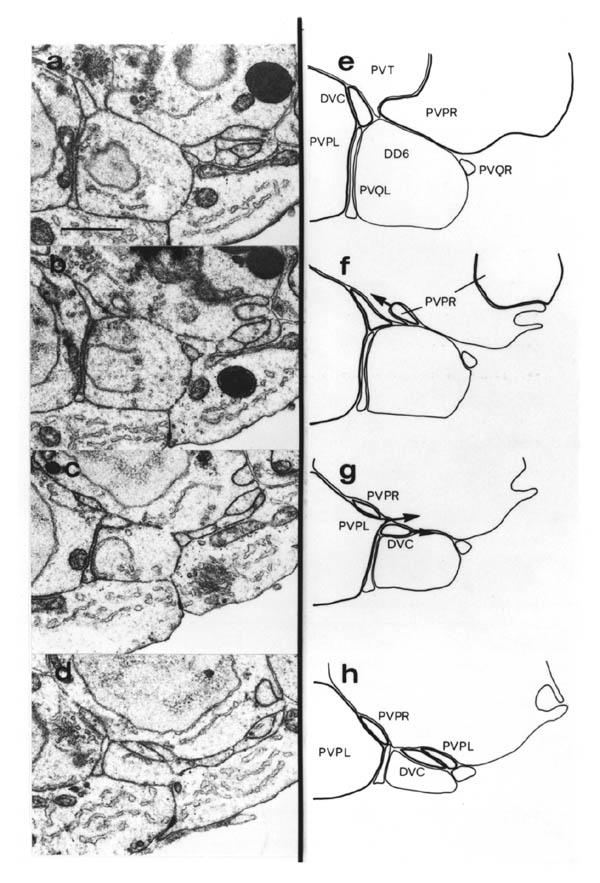 FIGURE 3.8. The crossover of PVP processes in the preanal ganglion (from the C reconstruction). (a) to (d) are a posterior to anterior series, each being separated from the next by about 0.5 microns. (e) to (h) are tracings of (a) to (d) showing the positions of significant processes. The sequence of events is as follows: at the front of its cell body (a) PVPR sends a process across to the left side (b), in front of which DVC and PVPL cross from left to right (c, d). The PVPL process invariably crosses in front of the PVPR process. Scale bar is 1 micron.
FIGURE 3.8. The crossover of PVP processes in the preanal ganglion (from the C reconstruction). (a) to (d) are a posterior to anterior series, each being separated from the next by about 0.5 microns. (e) to (h) are tracings of (a) to (d) showing the positions of significant processes. The sequence of events is as follows: at the front of its cell body (a) PVPR sends a process across to the left side (b), in front of which DVC and PVPL cross from left to right (c, d). The PVPL process invariably crosses in front of the PVPR process. Scale bar is 1 micron.
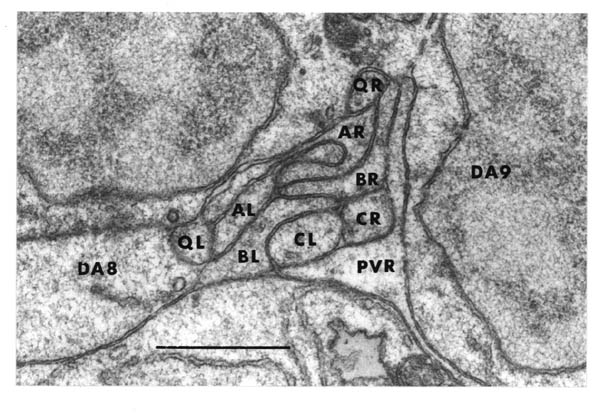 FIGURE 3.9. The place at the back of the preanal ganglion where the two lumbar commissures meet (from the C reconstruction). All the processes are labelled: QL/R are PVQL/R, AL/R are PHAL/R, BL/R are PHBL/R, CL/R are PVCL/R, and PVR, DA8 and DA9 are all the correct full names. The PHA, PHB and PVC processes all line up against each other, to some extent wrapping around their partners and thus increasing the area of contact (particularly the PHAL/R and thus increasing the area of contact (particularly the PHA processes). In contrast the PVQ processes appear to have no mutual affinity. More anterior to this, the PHA, PHB and PVC processes all grow along the right side together, while the PVQ processes split, one growing on he left and one on the right. Scale bar is 1 micron.
FIGURE 3.9. The place at the back of the preanal ganglion where the two lumbar commissures meet (from the C reconstruction). All the processes are labelled: QL/R are PVQL/R, AL/R are PHAL/R, BL/R are PHBL/R, CL/R are PVCL/R, and PVR, DA8 and DA9 are all the correct full names. The PHA, PHB and PVC processes all line up against each other, to some extent wrapping around their partners and thus increasing the area of contact (particularly the PHAL/R and thus increasing the area of contact (particularly the PHA processes). In contrast the PVQ processes appear to have no mutual affinity. More anterior to this, the PHA, PHB and PVC processes all grow along the right side together, while the PVQ processes split, one growing on he left and one on the right. Scale bar is 1 micron.
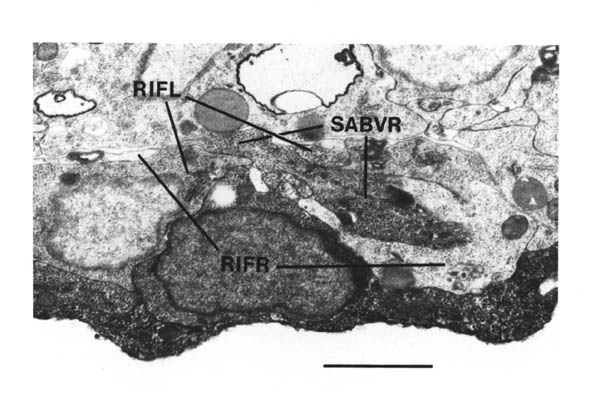 FIGURE 3.10.
The decussation in the retrovesicular ganglion (from the B series). The RIF processes have both just crossed over posterior to this section, and we can see the back of the SABVR cell body and its process also thrusting across the midline. In contrast to the preanal ganglion decussation here processes cross on the surface of the neuropil. Scale bar is 1 micron.
FIGURE 3.10.
The decussation in the retrovesicular ganglion (from the B series). The RIF processes have both just crossed over posterior to this section, and we can see the back of the SABVR cell body and its process also thrusting across the midline. In contrast to the preanal ganglion decussation here processes cross on the surface of the neuropil. Scale bar is 1 micron.
A phenomenon equivalent to the PVP crossover is seen in the retrovesicular ganglion at the front of the ventral cord, which is once again more symmetrical in the embryo than in the adult. There are three bilateral pairs of neurons in the embryonic retrovesicular ganglion: RIGL/RIGR, RIFL/RIFR and SABVL/SABVR, all of whose processes run forward. The RIF processes have grown out in the A and B reconstructions and once again they are seen to cross over by their cell bodies (figure 3.10). Following this observation, careful comparison of the positions of cell bodies in embryonic reconstructions and the embryonic lineage study (Sulston et al., 1983) with those in the adult reconstructions (White et al., 1986) confirmed that the RIF processes did indeed cross over in the adult reconstructions, and that the RIG and SABV processes do the same. The Processes from all three classes cross in nearly the same place in the adult reconstruction, by the SABV cell bodies. The SABV processes are just beginning to grow out and cross in the B reconstruction (figure 3.10), but the RIG processes have not yet grown out.
Therefore crossing over is seen in all the embryonic left/right pairs of interneurons in what might be termed the extended ventral cord, i.e. everything on the ventral hypodermal ridge between the excretory duct and the anus. During postembryonic development another interneuron pair, AVFL/AVFR, is added in the retrovesicular ganglion, but their processes are not bilaterally symmetrical; they are bipolar, running back together down the right side of the ventral cord and forward also together to the left of the excretory duct and round the left side of the nerve ring.
The crossing over, or decussation, of processes to the opposite side of the body from the soma is a property of many nerve types in higher animals, and the cases observed here may provide extremely simple examples of the same event that are susceptible to experimental manipulation of both the cells involved and of their environment. Several cell ablation experiments were performed to investigate factors involved in the PVP cross over in the preanal ganglion (Chapter 4).
3.7 Growth cone insertions into other cells
An observation previously made in other animals is the insertion of thin
processes from growth cones into other neuronal processes or target tissues
that might be important for guidance (Bastiani and Goodman, 1983). The
same phenomeno9n has been observed in the developing C. elegans nervous system, and there are two cases in particular where it is
especially noticeable and correlates with possible guidance decision
taking.
The first case is when the motor neuron commissures reach the dorsal
midline. In one reconstruction (wild type but not on of A to D), a single
commissure has just reached the dorsal hypodermal ridge, on emerging from
underneath the left dorsal muscle quadrant. The growing tip of this
commissure inserts two stubby finger-like processes about 0.1 - 0.2 microns
in diameter and 0.4 - 0.8 microns long into the dorsal hypodermal ridge
(figure 3.11). It is at this stage that the growth cone must turn through
a right angle and grow along the ridge.
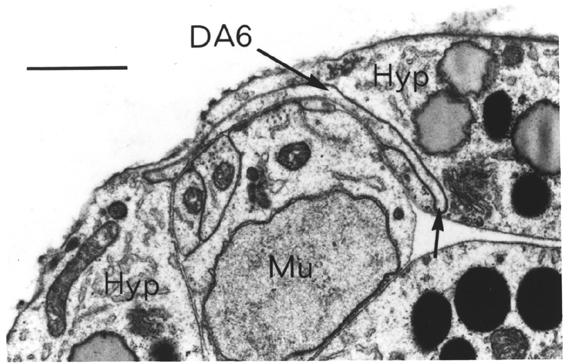 FIGURE 3.11. A finger from the DA6 commissural growth cone inserting into the dorsal
hypodermal ridge as it comes out from under the left dorsal muscle
quadrant. The particles to the left of the finger are not vesicles
(determined by viewing at higher resolution).
FIGURE 3.11. A finger from the DA6 commissural growth cone inserting into the dorsal
hypodermal ridge as it comes out from under the left dorsal muscle
quadrant. The particles to the left of the finger are not vesicles
(determined by viewing at higher resolution).
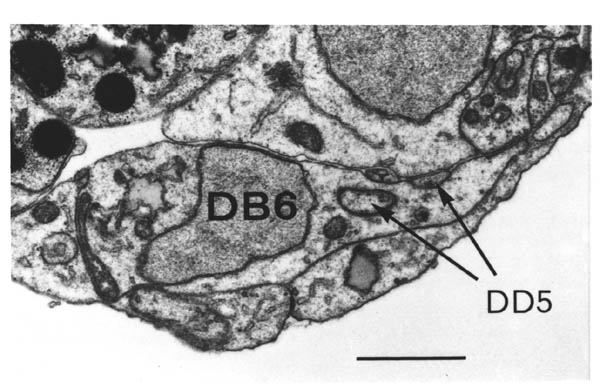 FIGURE 3.12. An insertion of an extension at the front of the DD5 ventral cord process into the cell body of DB6. DD5 stops growing forward along the cord at about the point that it reaches DB6. Both DD5 and DB6 send commissures out to the right around the same place. A part of the DB6 commissure can be seen. From the C construction. Scale B 1 micron.
FIGURE 3.12. An insertion of an extension at the front of the DD5 ventral cord process into the cell body of DB6. DD5 stops growing forward along the cord at about the point that it reaches DB6. Both DD5 and DB6 send commissures out to the right around the same place. A part of the DB6 commissure can be seen. From the C construction. Scale B 1 micron.
The second case concerns what happens when DD processes meet DB cell bodies. In all cases that have been reconstructed the
anterior tips of DD6 and DD5 insert into the bodies of DB7 and DB6 respectively. Insertion of DD4, DD3 and DD2 into DB5, DB4 and DB3 also takes place but less frequently and in a less pronounced
fashion. An example of DD5 insertion into DB6 is shown in (figure
3.12). These DD insertions into DB cells reinforce the suggestion
made above that DB cells might be involved in DD morphology.
Where the insertions are most pronounced (DB6 and DB7), the DB commissures grow to the right next to the DD commissures.
There are occasional other insertions into the ventral hypodermis
from growth cones of processes growing along the ventral cord
(data not shown), but no particular pattern is discernible. Also
I have seen no cytological correlates of the insertions, such as
vesicles clustering around the insertion in the cell into which
the insertion is made, which have been seen in the corresponding
phenomenon in insects (Bastiani and Goodman, 1983).
Chapter 4: Laser Ablation Experiments
4.1 AVG
4.2 DD3/5
4.3 PVP and PVQ
4.4 DVC
The previous chapter described a times series of
embryonic
reconstructions that allowed a picture to be drawn of the course
of normal nerve outgrowth in and around the ventral nerve cord.
This chapter describes a set of cell ablation experiments in which
chosen cells were removed by ablating their parents with a
focussed laser beam, using a system developed by J. G. White
(Sulston and White, 1980). Chapter 2 describes the protocol used.
The advantage of killing the parent cell is not only that it
unequivocally prevents production of the cell of interest, but
also that the remains of the dead cell are excluded from the
embryo when the ventral hypodermis closes up, removing them also
from any possible influence. The chapter is organised with a
section for each set of experiments.
4.1 AVG
AVG is the first process to grow along the ventral cord
(Chapter 3). It grows back along the right hand side, and later
the adult cord is remarkably asymmetrical, with over 90% of its
processes on the right hand side (there are 3 to 5 on the left,
depending on anterior/posterior position, as against about 50 on
the right). To what degree is AVG involved in establishing this
asymmetry, and which cells, if any, depend directly on AVG to
correctly determine the positioning of their processes? To answer
these questions I removed AVG by ablating its parent cell,
Abprpapppa. The sister of AVG,
which is also removed by this
ablation, is RIR,
a ring interneuron whose cell body and processes
all lie some distance anterior to those of AVG,
and whose synaptic
connections in the adult are not closely related to ventral cord
circuitry (White et al., 1986).
Nine experimental
animals
were permitted to develop and hatch in order to test whether motor
control was affected, a crude test of ventral cord function. Six
of these showed a very mild uncoordinated phenotype as newly
hatched L1 larvae, in some cases only clearly visible when the
worm was made to swim in water, in which case sections of their
thrashing bodies looked stiffer than normal. Those worms that
were uncoordinated as larvae were similarly uncoordinated as
adults.
One of the adults showing an uncoordinated
phenotype was sectioned through the front part of the ventral cord
and the retrovesicular ganglion. Eileen Southgate reconstructed
this series, since she and John White were interested in another
question, concerning regulation in the circuitry.
The
reconstruction confirmed that AVG was absent sine: (1) there were
one too few cells in the RVG, and (ii) there were only two cells
in the RVG with posteriorly directed processes that extended back
through the complete series (AVFL and AVFR,
they and AVG are the
only ventral cord interneurons in the RVG). Almost all the cells
in the RVG were identifiable, but there were some ambiguities
concerning motor neuron identification both here and in the
anterior ventral cord. This is because the spatial organisation
of many nerve processes, especially those belonging to motor
neurons, was abnormal. Cell body positions tended to be slightly
displaced from normal, but the general order was preserved.
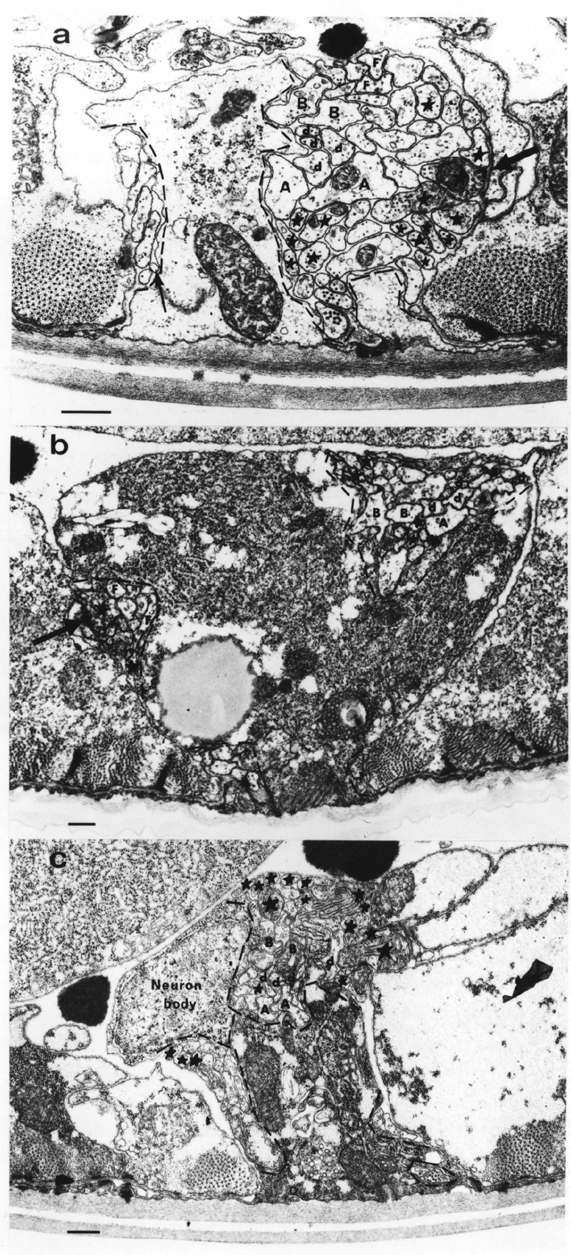 FIGURE 4.1. Adult ventral cords from (a) a normal animal, (b) an animal in which AVG had been removed, (c) an unc-3 mutant. The hypodermal ridge is
raised in adults compared with embryos. Process bundles are outlined
in
dashes. There is only one on each side of (a), but there are four
bundles
in each of (b), (c), with many more processes (labelled with stars)
are on
the right, but in (c), (c) there are also motor neurons on the left.
There
is a neuromuscular junction on the right in (a), and one from DB3 on the left, abnormally, in (b) (thick arrows). The motor circuitry
interneuron processes are labelled A for AVAL/R , B for AVBL/R , and d for AVDL/R and AVEL/R , which are indistinguishable in this part of the cord. They are
abnormally rotated, with motor neuron processes on the hypodermal side
of
them in (b). In (a), (b) the two AVF neurons are labelled F. They are on the left in (b), which is not
normal.
The thin arrow in (a) points to a hypodermal extension, rather than a
neuronal process. Scale bars are 1 micron in each case.
FIGURE 4.1. Adult ventral cords from (a) a normal animal, (b) an animal in which AVG had been removed, (c) an unc-3 mutant. The hypodermal ridge is
raised in adults compared with embryos. Process bundles are outlined
in
dashes. There is only one on each side of (a), but there are four
bundles
in each of (b), (c), with many more processes (labelled with stars)
are on
the right, but in (c), (c) there are also motor neurons on the left.
There
is a neuromuscular junction on the right in (a), and one from DB3 on the left, abnormally, in (b) (thick arrows). The motor circuitry
interneuron processes are labelled A for AVAL/R , B for AVBL/R , and d for AVDL/R and AVEL/R , which are indistinguishable in this part of the cord. They are
abnormally rotated, with motor neuron processes on the hypodermal side
of
them in (b). In (a), (b) the two AVF neurons are labelled F. They are on the left in (b), which is not
normal.
The thin arrow in (a) points to a hypodermal extension, rather than a
neuronal process. Scale bars are 1 micron in each case.
The most striking aspect of the process bundle
disorganisation can be seen in a random cross section of the cord
behind the RVG (figure 4.1): instead of a large bundle of about 50
processes on the right and one of 4 or 5 on the left there are
several smaller bundles, including two on the left hand side.
There is no fixed arrangement of these small bundles as one
progresses along the cord: processes occasionally transfer between
bundles, and sometimes bundles fuse to form a larger grouping or
split to form two smaller ones. However there are always
significantly more processes on the right than on the left. The
total number of processes appears normal (this comparison can only
be made approximately, since the number of motor neuron processes
present at any particular point is variable).
Several of
the motor neurons are disrupted. These are motor neuron cell
bodies associated with both sides of the cord (VD2 and DB3 are to
the left), and also motor neuron processes on both sides (all of VD3 and parts of DB3, VD2 and VB2 processes are on the left). In
addition DB3 is lacking a commissure, and DD2's
commissure is
severely misplaced or missing (the DD2 cell body is off the
posterior end of the reconstruction, but its commissure should
come out from the cord with that of DA2,
some 150 sections
anterior to the end of the series). Instead of a commissure, the DB3 process has a branch that crosses to the left hand side and
shows some characteristics of the normal dorsal branch, in that
first it runs backwards from the crossover point, and second it
contains three neuromuscular junctions (figure 4.1). Normally all
ventral cord neuromuscular activity is from the right hand cord,
and all DB3's
neuromuscular output is from its backward dorsal
branch.
Many of the interneuron processes cannot be
identified because they make no synapses and their cell bodies are
outside the bounds of the reconstruction. Among those that can
are the two AVF neurons, which have cell bodies in the RVG, and
which both send their processes back down the left hand cord in
this reconstruction, as opposed to the right normally (figure 4.1). It is also possible to identify the 8 main motor circuitry
interneurons by class (2 each of AVA and AVB,
and the 4 AVE neurons, figure 4.1), because of their patterns of synaptic output
and gap junction formation with the motor neurons. In most cases
where they are accessible to the motor neurons the normal synaptic
connections are made. Normally these motor circuitry interneurons
run in the central left side of the main (right hand) ventral
cord, with a regular internal order: AVB's
on top, AVA's
on the
bottom, and AVD's
and AVE's
loosely sandwiched in between. In
this reconstruction they all keep together in the main right hand
bundle, and amongst themselves they roughly preserve their normal
order, but the whole group is often displaced from its regular
position and orientation (figure 4.1). Thus it appears that, as a
group, their internal organisation remains, but that they have
lost the external cues that give the group as a whole a fixed
position relative to other processes, some of which, indeed, are
separated by being in other bundles.
In addition to this
adult reconstruction I also looked at an embryo in which the
parent of AVG had ablated, fixing it at the stage when the motor
neuron commissures are normally just growing out from the ventral
cord (around 500 minutes). In this case I reconstructed the back
part of the ventral cord and also the preanal ganglion (PAG).
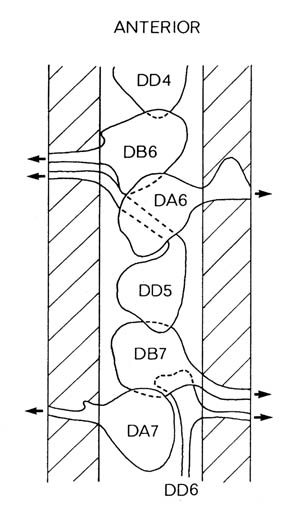 FIGURE 4.2. A schematic illustration of the reconstruction of the ventral cord
from the
embryo in which AVG had been removed. The illustration has the same form as the central
region
of the diagrams in figure 3.4. There is no continuous interneuron
process
in the cord, indicating that AVG was both correctly identified and correctly removed. The positions where
commissures are leaving the ventral cord are indicated by arrows. The DD6, DA7 and DB7 neurons look normal, but the DD5 ventral cord process switches from right to left, and all the commissures
from DD5, DB6 and DA6 are leaving the cord from the wrong side (compare with figure 3.5).
FIGURE 4.2. A schematic illustration of the reconstruction of the ventral cord
from the
embryo in which AVG had been removed. The illustration has the same form as the central
region
of the diagrams in figure 3.4. There is no continuous interneuron
process
in the cord, indicating that AVG was both correctly identified and correctly removed. The positions where
commissures are leaving the ventral cord are indicated by arrows. The DD6, DA7 and DB7 neurons look normal, but the DD5 ventral cord process switches from right to left, and all the commissures
from DD5, DB6 and DA6 are leaving the cord from the wrong side (compare with figure 3.5).
Although the AVG process was clearly missing, there was no
other alteration to the organisation of the PAG and the early
posterior interneurons that grow forward along the cord from it
(the PVP's, PVQ's, DVA and DVC),
every process following its normal
trajectory. However, as in the adult, the ventral cord was
disorganised. In this case the most posterior three motor neurons
(DD6, DA7 and DB7)
looked normal, but DD5's
anterior process in
the ventral cord, although leaving the cell body on the right side
as normal, switched sides from right to left and sent out its
commissure on the left. DB6,
whose commissure usually goes to the
right with that of DD5,
sent it s commissure to the left also. DA6's
commissure, which is usually on the left, went to the right
instead (figure 4.2).
In summary, it seems that, in the
absence of AVG,
the motor neurons in the ventral cord are variably
disorganised in terms of process growth. Some examples look
normal, while others send processes on the wrong side of the cord,
or fail to form commissures, etc. This applies to both embryonic
and postembryonic motor neuron classes, although the postembryonic
neurons look much less affected. A second, possibly related,
consequence of AVG removal is a splitting of the ventral cord into
several bundles, some of which are on the left hand side of the
cord. Some interneurons are also split off into these alternative
bundles, but in the adult example that was reconstructed the main
motor circuitry interneurons look fairly normal. Many of the
motor neurons are able to make correct synaptic contact both with
their innervating interneurons and with muscle. This probably
explains why the observed behavioural phenotype of removing AVG was only minor uncoordination, when a difference was noticeable at
all.
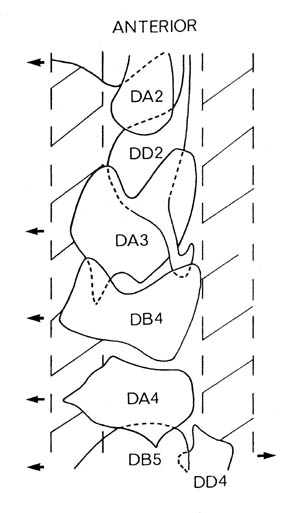 FIGURE 4.3. A schematic illustration of the same form as figure 4.3 of the ventral
cord
reconstruction of the embryo in which the DD3/DD5 parent had been ablated. DD3 is missing (see figures 3.4, 3.5 for the comparable region in normal
animals). The DD2 process has grown slightly back but has not grown beyond the front of DB4, while DD4 has not grown forward beyond DB5. There is thus a gap of an entire cell between the DD processes.
However since this is a young embryo (approx. 175 minutes) one cannot
say
if the gap will be filled later.
FIGURE 4.3. A schematic illustration of the same form as figure 4.3 of the ventral
cord
reconstruction of the embryo in which the DD3/DD5 parent had been ablated. DD3 is missing (see figures 3.4, 3.5 for the comparable region in normal
animals). The DD2 process has grown slightly back but has not grown beyond the front of DB4, while DD4 has not grown forward beyond DB5. There is thus a gap of an entire cell between the DD processes.
However since this is a young embryo (approx. 175 minutes) one cannot
say
if the gap will be filled later.
4.2 DD3/5
In the wild type embryo, after the growth of AVG back along the right hand cord, the DD motor neurons grow out processes in the ventral cord next to AVG. These processes
grow forward until they meet or almost meet the next DD in the sequence (in the various
wild type embryonic series they were often separated by a gap of about 1 micron, Chapter
3). Then commissures grow out to the right from near their front tips. By removing a DD cell and examining whether the processes would extend further along the cord to fill
in the gap, I was able to test whether process growth is terminated solely by contact, and
if so, whether the position of the commissure also changed. Does it always leave from the
front of the ventral cord process?
In fact it was easy to
remove DD3 and DD5 together, creating two gaps in a single animal,
since they are sisters. As with AVG it was checked that their
dead parent (Abplppappp) was excluded from the embryo after laser
ablation, and that the relevant DD cell was missing in the subsequent reconstruction.
I have reconstructed the front part of one embryo from the seven that were fixed and
sectioned. In this animal, which is the same age as the wild type A series (around 480
minutes), the gap left by removing DD3 has not been
filled by DD4.
Instead the DD4 process stops at the front of DB5,
only very slightly further forward, if at all, than normal (figure
4.3). There is a short posterior extension from DD2,
which is not
unusual (figure 3.5), but this stops around DB4,
leaving a gap
with no DD processes along the whole
extent of the DA5 cell body.
4.3 PVP and PVQ
The third set of ablation experiments concern the four PVP and PVQ neurons. To summarise briefly: these form the first group of interneurons
to grow forward from the back of the ventral cord. The PVQ cell bodies are in the lumbar ganglia; they send processes down the lumbar
commissures through the preanal ganglion (PAG), where they pick up
contact
with the PVP processes, and then forward along the ventral cord, one on each side. The PVP bodies lie in the PAG; their processes leave their bodies heading towards
the midline, cross over, and then grow forward on the opposite side of
the
ventral cord. So PVPR runs with PVQL on the left hand side, while PVPL runs with PVQR on the right. The growing tips of each PVP/PVQ pair, either on the left or right, are always very close (within 0.5
microns). The only processes apart from PVPR and PVQL to grow down the left side of the ventral cord in the embryo are AVKR and RMEV,
both of which grow back from the front, RMEV stopping part back. In the adult the left vulval motor neuron HSNL also grows forward on the left side of the cord from the vulva half way
along the body. In the oldest wild type embryonic series (D series)
only
one of AVKR and RMEV was seen growing back in the anterior cord (it is not knows which one),
and
this was only after PVPR and PVQL had reached the front.
The questions that can therefore be asked concerning possible
organising
roles for PVP and PVQ processes are:
1. Are one or both of a PVP/PVQ pair needed for the other to grow along the cord?
2. Are PVPR and PVQL needed for growth of the other processes down the left cord?
3. Are the PVQ processes, or the other PVP cell, necessary for crossing over of the PVP processes in the preanal ganglion?
4. Is the growth of a PVQ process down a lumbar commissure necessary for other lumbar ganglion cell
processes on the same side to reach the preanal ganglion?
Experiments were carried out in which PVPR, PVPL and PVQL were independently removed. As with AVG and DD3/DD5,
a block of fixed experimental embryos was completely sectioned for each of
the sets of ablations (7 embryos for PVPR,
5 for PVPL,
and 5 for PVQL). In addition 5 adult PVPR experimental animals were cut at 3 random sites in the posterior half of
the cord to help answer the second question. Again the parent cell
was
ablated in each case and only embryos that excluded the dead cell on
closure of the hypodermis were considered further. In the case of
PVQL the
parent is ABplapppa and the sister cell normally undergoes programmed
cell
death and engulfment soon after being born; therefore there is no
additional cell missing in experimental animals at the time of process
outgrowth. The sisters of PVPL and PVPR (parents AB.plppppa and AB.prppppa)
are left and right ventral rectal epithelial cells (repVR and repVL).
Together with repD these form a ring of rectal cells that lie above
and
forward of the PAG; they are sufficiently distant to be unlikely to be
important in nerve process guidance in the PAG. In the reconstructions
of PVPR and PVPL experimental embryos the correct repV cell was seen to be missing; in each
case the rectum had resealed by extension forward of one of the
posterior
neighbouring pair of rectal epithelial cells (K and K') rather than
circumferential filling in by the other rep cells.
I will consider the four questions posed in above in turn:
1. It is easier to observe the presence or absence of PVP/PVQ processes on the left side of the cord (PVPR and PVQL) than on the right side, since during the stages under
consideration those are the only nerve processes on the left side. I
first
considered the embryos in which PVPR had been removed. Of the four embryos in which it was possible to
identify a region of the ventral cord anterior to PAG where the PVPL and PVQR processes were visible on the right side of the cord, none had
any
processes on the left side (figure 4.4). The PAG and posterior cord
of one
of these embryos was reconstructed; in this case PVQL grew forward as
normal through left side of the PAG past the point where it would
normally
have picked up contact with PVPR and then, at the front of DD6,
which was displaced slightly anterior to its normal position, it switched
sides from left to right and ran forward for a short distance with PVPL and PVQR (figure 4.5). Its anterior tip, however, was more than 2.5
microns posterior to the tips of PVPL and PVQR (which were off the anterior end of the series, 54 sections from
the PVQL tip). Therefore it appears that PVPR is necessary for growth of PVQL along the left side of the cord, and that
in its absence PVQL is retarded somewhat, but grows forward along the
established path of PVPL and PVQR.
However, when PVPRL (erratum in the manuscript) was removed, in each
of
the three embryos for which the same region anterior to the PAG was
identified, a solitary process was seen on the left side (figure
4.4). The
PAG region of two of these embryos was reconstructed; in each case
PVQL was
missing and PVPR grew as normal along the left side of the cord. In the younger of the
series (about 470 minutes) it stopped about 1.7 microns (34 sections)
posteriorly to the point where the PVPL/PVQR processes on the right stopped; the older series did not contain the
anterior tips of any of the processes. Therefore, in contrast to
PVQL, it
appears that PVPR is competent to grow by itself to the left side of the cord.
Finally, I considered the consequences of removing PVPL,
the bilaterally homologous experiment to that of removing PVPR.
In this case PVQR stayed on the right side, rather than crossing to join PVPR and PVQL (two animals: one was reconstructed completely and one animal had
two long processes but no third process in the left cord, even near
the
PAG, figures 4.4, 4.5). However it must be remembered that, in the
absence
of PVQL when PVQR was removed, since event though the PVPL process is absent on the right side of the cord there are still AVG and PVPR was removed there was nothing on the left side. The corresponding
reciprocal experiment of removing PVQR was not attempted, since it
seemed
unlikely that there would be an effect in the more populated right
hand
side of the cord, where there had been none when PVQL was removed on
the
left side.
In summary, PVPR is necessary for growth of PVQL on the left side of the cord. In its
absence PVQL grows on the right side. However PVPL is not necessary for PVQR to grow on the right side, presumably because
PVQR can follow the preexisting AVG and DD processes there. In contrast, the removal of PVQL has no significant effect on PVPR.
2. To answer the question of whether PVPR and PVQL are needed for growth of other processes down the left cord, I
ablated the parent of PVPR in six animals and looked at the left side of the adult, rather than the
embryonic, so that all processes would have had the opportunity to
complete
growth. The fixed animals were cut at three random sites in the
posterior
half of the body, where AVKR is normally present on the left side together with PVPR and PVQL. One of the five animals was rejected because of poor fixation.
None of the remaining five had any consistent process showing on the
left
side of the cord (figure 4.4). In several cases there appeared to be
a
process visible at one of the sites. This was probably a fold or
finger of
hypodermis; such hypodermal extensions are common around the adult
ventral
cord (for example there are two in the section from the control
reconstruction in figure 4.1). Therefore both PVPL,
as expected from the previous result, and AVKR were missing from the left side in all five cases, implying that the PVPR/PVQL pair is necessary for AVKR to grow down the left hand ventral cord. It is not possible to ascertain
whether AVKR had switched to the right side of the cord in the experimental animals, or
had failed to grow back at all, without reconstruction of the complete
nerve ring.
3. The removal of neither a PVP nor a PVQ cell affected the crossing over the opposite side of the PVP processes when they leave their cell bodies in the centre of the preanal
ganglion (figure 4.5). The embryonic PAG reconstructions after
removing
either PVPR or PVPL show that the remaining PVP cell sent its process across the midline in exactly the same location as
usual. This rules out an explanation of the chiasm being caused by
mutual
attraction of PVP processes. The reconstructions after PVQL parent ablations also showed no
change.
4. However there did appear to be an effect on the left lumbar
commissure
when PVQL was removed. In neither of the two experimental animals
that
were reconstructed did any other processes come down the left lumbar
commissure into the PAG, although in each case the DA8 process had already grown dorsally via the same path out of the PAG. One
of
the reconstructed animals was young enough that the following
processes on
the right side had only just passed through the commissure; however in
the
second four of the right hand lumbar processes other than PVQR had
grown
half way through the PAG (figure 4.6).
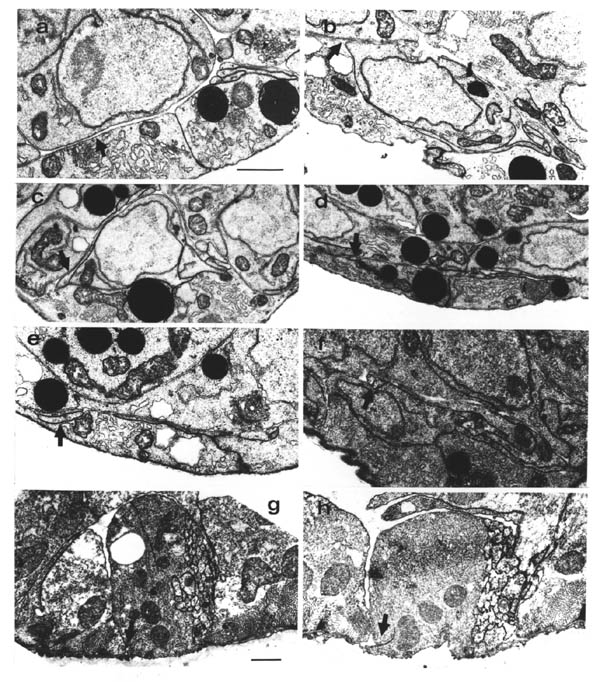 FIGURE 4.4. The ventral cords of animals in which a PVP or PVQ cell has been removed. In each case an arrow points to the left hand
cord.
For embryonic cords compare with figure 3.7 (b) for a control, and for
adult cords compare with figure 4.1 (a). Two examples of each
experiment
are shown. (a), (b) Embryonic cords after removal of PVPR; there are no processes on the left side, but sufficiently many on the
right to show that PVQL would normally have been visible in these
situations. (c), (d) Embryonic cords after removal of PVQL; there is one process on the left side. (e), (f) Embryonic cords after
removal of PVPQ (NB, typo); 2 processes on the left. (g), (h),
Posterior
adult cords after PVPR removal; there are still no processes on the left side in the posterior
half of the animal. Scales bars in (a) for (a) to (f), and in (g) for
(g),
(h), 1 micron in each case.
FIGURE 4.4. The ventral cords of animals in which a PVP or PVQ cell has been removed. In each case an arrow points to the left hand
cord.
For embryonic cords compare with figure 3.7 (b) for a control, and for
adult cords compare with figure 4.1 (a). Two examples of each
experiment
are shown. (a), (b) Embryonic cords after removal of PVPR; there are no processes on the left side, but sufficiently many on the
right to show that PVQL would normally have been visible in these
situations. (c), (d) Embryonic cords after removal of PVQL; there is one process on the left side. (e), (f) Embryonic cords after
removal of PVPQ (NB, typo); 2 processes on the left. (g), (h),
Posterior
adult cords after PVPR removal; there are still no processes on the left side in the posterior
half of the animal. Scales bars in (a) for (a) to (f), and in (g) for
(g),
(h), 1 micron in each case.
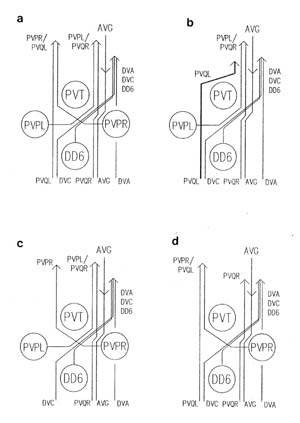 FIGURE 4.5. Schematic diagrams of the front of the preanal ganglion in normal
embryos
and ones in which a PVP or PVQ cell has been removed, based on complete reconstructions of the
preanal
ganglia in these animals. The ages of the reconstruction varied but
they
were all around 500 minutes. (a) normal, (b) after PVPR removal; (c) after PVQL removal, (d) after PVPL removal. Neither of the last two experiments caused any effect on other
processes in this region.
FIGURE 4.5. Schematic diagrams of the front of the preanal ganglion in normal
embryos
and ones in which a PVP or PVQ cell has been removed, based on complete reconstructions of the
preanal
ganglia in these animals. The ages of the reconstruction varied but
they
were all around 500 minutes. (a) normal, (b) after PVPR removal; (c) after PVQL removal, (d) after PVPL removal. Neither of the last two experiments caused any effect on other
processes in this region.
4.4 DVC
One further ablation experiment was tried in an attempt
to
understand why the PVP processes cross over in the preanal
ganglion. As described in Chapter 3, at the point where the
crossover takes place the process of DVC spreads out into a thin
sheet that separates the cell bodies of PVT and DD6;
the PVP processes actually cross between PVT and the DVC sheet. It
therefore seemed possible that DVC was essential for the
crossover. Therefore five embryos were fixed in which the parent
of DVC had been ablated, two of which were later reconstructed in
the region of the PAG.
The sister of DVC (parent Caapa)
normally undergoes programmed cell death before differentiating
and so is unlikely to be required for the development of the PAG.
Since the DVC parent lies underneath the tail hypodermal cells at
the time of its ablation it is not excluded from the embryo as in
all other cases. However I checked that condensed nuclear debris
was visible about 20 minutes after the ablations, and the absence
of the DVC cell body was confirmed in the two reconstructed
embryos.
No effect was seen on thePVP crossover in either
reconstruction. Instead PVQR and PVQL flattened out somewhat and
met in the centre, partially replacing DVC's
role in separating PVT and the crossing over PVP's
from DD6 (figure 4.7). This
possibly suggests that PVQL, PVQR and DD6 have somewhat
interchangeable or redundant functions at this point in organising
the PAG. However the multiple ablations which might test this
suggestions have not yet been attempted.
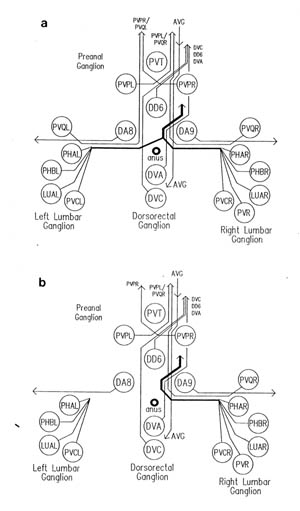 FIGURE 4.6. The complete posterior nervous system in 500 minute embryos, shown as
in figure 1.3. (a) Normal, (b) after PVQL removal. Although there was no effect on the PVP processes in (b) (see figure 4.4), the other processes from the left
lumbar
ganglion have failed to grow down the left lumbar commissure. However
the DA8 process, which also grows in the lumbar commissure, but in the opposite
direction looks normal.
FIGURE 4.6. The complete posterior nervous system in 500 minute embryos, shown as
in figure 1.3. (a) Normal, (b) after PVQL removal. Although there was no effect on the PVP processes in (b) (see figure 4.4), the other processes from the left
lumbar
ganglion have failed to grow down the left lumbar commissure. However
the DA8 process, which also grows in the lumbar commissure, but in the opposite
direction looks normal.
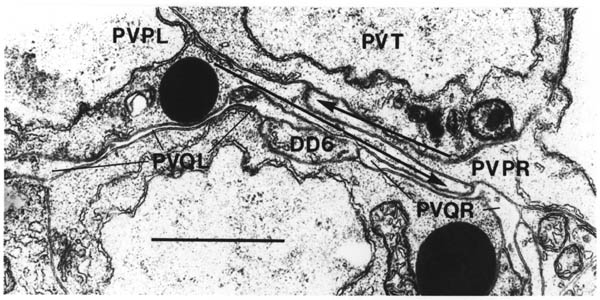 FIGURE 4.7. The site of PVP process crossover in an embryo in which DVC has been removed. The PVP processes still cross over (thick arrows). However the situation is a
little abnormal because PVQR flattens out much more than normal (compare with figure 3.8). Scale bar
is
1 micron.
FIGURE 4.7. The site of PVP process crossover in an embryo in which DVC has been removed. The PVP processes still cross over (thick arrows). However the situation is a
little abnormal because PVQR flattens out much more than normal (compare with figure 3.8). Scale bar
is
1 micron.
Chapter 5: Discussion
5.1 Reliability
5.2 The asymmetry of the ventral cord
5.3 Motor neuron outgrowth and formation of the dorsal cord
5.4 Discussion
5.5 Selective fasciculation
5.6 Conclusion
The last two chapters have described the results of a number of electron
microscope reconstructions of the developing ventral nervous system in both
normal and experimental C. elegans embryos. In a short space of
around an hour the first nerve process grows back along the ventral cord
from the front, the motor neurons in the ventral cord grow commissures
around the body of the animal to form the dorsal cord, and a number of
additional processes grow forward from the preanal ganglion at the back of
the animal. The short time taken in laying down the skeleton of the
ventral nerve cord and preanal ganglion reflects the rapid development of
C. elegans embryos (13 hours total). The small number of cells present,
and the simple morphologies of the nerve cells, allow precise suggestions
to be made about the roles of individual cells during process outgrowth.
Several possible intercellular interactions were investigated by killing
the parents of specific cells with a focussed laser beam. Before
discussing the pattern of process outgrowth in the ventral nervous system,
and how it might be controlled, I will first consider the reliability of
the observations on which the work is based.
5.1 Reliability
The approach of reconstruction from serial electron micrographs precludes
the examination of a large number of individual animals, either in the wild
type time series or in any particular experiment. It is reasonable to ask
whether reliable conclusions can be drawn from the necessarily small number
of reconstructed animals that have been presented: there are two possible
sources of error or variation: experimental "noise" created by variability
in the observational and experimental techniques, and natural variability
of the phenomena themselves.
As far as the determination of process disposition is concerned the
technique is very reliable; each individual reconstruction provides a large
amount of information at a very fine level of detail, so that essentially
all the nerve processes present can be positively identified and a complete
picture of the relevant parts of each neuron determined (the exceptions are
discussed in Chapter 2). The technique of laser ablation of individual
identified cells is also very specific. It is unlikely that the killed
cell has any residual influence, because, except for the DVC parent ablation, when no subsequent change was seen in any other cells
anyway, the dead cell was observed to be excluded from the embryo when the
hypodermis closed up (figure 2.2). It is in principle possible to damage
neighbouring cells at the time of ablation, and in fact one of the embryos
sectioned in the DD3/DD5 set showed signs of general morphological disorganisation, presumably due
to such damage. However in all the cases discussed, except the AVG experiments, any changes that were observed were confined to a small number
of neurons normally associated with the particular missing cell. Although
control experiments in which random neighbouring cells were ablated were
not performed, altogether six different cells all near together on the
ventral surface of the 270 minute embryo (figure 2.1) were ablated without
there being any overlap in the observed consequences.
As regards intrinsic variation, all the reconstructions are consistent with
a fixed time sequence of normal axonal outgrowth. When a change in this
pattern was seen in laser ablation experiments then, again excepting the AVG experiments, it was clean and restricted in its extent, was generally
observed in at least two cases, and was consistent, never being seen in one
case but not another. The situation with respect to the removal of AVG appeared to show variability and is discussed more fully in the next
section. However, taking all the results together and in conjunction with
the known fixed adult anatomy, there is sufficient evidence to indicate
that the developing C. elegans nervous system is simple and
reproducible enough for the techniques used here to provide an accurate
picture of events.
The high level of reproducibility and the generally restricted, fixed
effect of removal of individual cells are typical of C. elegans development and anatomy. The cell lineage and the disposition of somatic
cells at all stages of development are known to be nearly invariant
(Sulston and Horvitz, 1979, Kimble and Hirsh, 1979, Sulston et al.,
1983) the final anatomy is equally stereotyped (White et al., 1986).
Although a number of cases of adjustment in cell lineage after individual
cell ablations are known (e.g. Sulston and White, 1980, Sulston et al.,
1983) they are the exception rather than the rule, and in no case do they
result in complete regulation back to the native form. All the results of
ablation experiments performed here are consistent with only the daughters
of the ablated cell being missing, and with there being no change of
identity of any other cell. In addition they confirm the relevant cell
assignments in the embryonic lineage, since in each case only the expected
cell or cells was or were missing.
5.2 The asymmetry of the ventral cord
One of the striking features of the C. elegans ventral nervous
system is the almost, but not quite, complete asymmetry of the ventral
nervous cord, which has around 55 neurons on the right side and only 4 or
so on the left. If all the processes were together on the right hand side
then it could be regarded as a single fused nerve that was displaced to one
side for steric reasons, but since a small number of left/right pairs of
processes are arranged symmetrically (PVQ, PVP, AVK and in the adult, HSN)
the question arises of why not all the others? In fact the arrangement is
essentially symmetrical anterior to the RVG; the cord splits into two to
pass the excretory duct on both sides, with each bilateral pair of
processes being split so there is one member on each side, an stays
symmetrical throughout the ventral ganglion and into the bottom of the
nerve ring.
Most animals with a symmetrical body plan have a symmetrical ventral
nervous system, often consisting of a chain of ganglia linked by paired
nerves, which are sometimes fused but clearly retain their symmetrical
character. There are in fact some nematodes that have symmetrical paired
ventral cords (Martini, 1916). Chitwood and Chitwood (1974), in discussing
the differences amongst nematode species, state (p. 162):
Differences in the central nervous system lie chiefly in the degree of
subdivision of the lateral ganglia, the form of the ventral ganglia, and
the degree of fusion of the ventral nerves.
They go on to state that in many species both around the RVG and for some
distance anterior to the PAG there are symmetrical paired nerves, though in
most cases these are fused for the main part of the length of the body.
They continue (p. 163):
The apparent doubleness in both anterior and posterior ends of the ventral
nerve caused Meissner and many later authors to conclude that the entire
nerve was at one time double... (we) subscribe to the primitive double
ventral nerve hypothesis.
Several observations that have been made in this study are relevant to the
origin of cord asymmetry. Perhaps I should start with AVG. AVG is a unique neuron with its body in the RVG, it is the first neuron to send
a process out along the ventral cord, and it sends it along the right hand
side. When AVG was removed by ablating its parent the cord was seen to be disrupted in two
ways.
First, as seen most clearly in the embryonic AVG reconstruction, the organisation of the embryonic ventral cord motor
neurons was disturbed. In particular a DD process was seen to
switch across to the left side of the cord and send its commissure round to
the left rather than the right, and the DA and DB cells near this
point also sent their commissures round the opposite side to normal. The
switch of the DD process to the left
cord confirms that AVG must normally grow out before the DD ventral cord
processes. In the adult AVG reconstruction the DB3 and DD2 cells show abnormal process organisation. These effects would seem to be a
direct consequence of the absence of AVG,
because the outgrowth of DD processes and motor
neuron commissures follow directly after the outgrowth of AVG.
The postembryonic motor neurons do not seem so badly affected as the
embryonic neurons, although the VD3 process in the adult reconstruction is switched from being on the right
side to the left.
The second effect of removal of AVG,
seen in the adult reconstruction, is a general disorganisation of the cord
in which instead of a large ordered bundle on the right side and a very
small one on the left there are several intermediate sized bundles at
various positions on the left and right sides (figure 4.1). This indicates
that AVG is ultimately necessary for correct organisation of the interneurons as
well as motor neurons, whose disarray appears earlier. However AVG does not seem to be necessary for outgrowth of processes, since the total
number of processes in a cross section of the experimental adult cord is
within the expected range, and all the fully reconstructed cells send out
processes in the correct direction, if not on the correct side.
It is also clear that AVG is not the sole determining influence for the left/right organisation of
the ventral cord, because in the embryonic experimental reconstruction all
the early interneurons from the back were growing forward correctly PVQR, PVPL, DVA and DVC on the right, and PVQL and PVPR on the left). Also in the adult AVG reconstruction the majority of processes was at all times on the right,
including the motor control interneurons (AVAL/AVAR, AVBL/AVBR, AVDL/AVDR and AVEL/AVER).
In the only positively identified case of interneurons growing on the
wrong side, both AVF's
were seen to grow on the left (they are normally both on the right, figure
4.1).
The fact that removal of AVG leads to no major behavioural defect suggests that it has no critical
function of its own. In the adult reconstruction, although it is a fairly
large cell, it has been seen to make very few connections to other neurons,
the only consistent ones being large gap junctions to the two RIF interneurons and a small amount of synaptic input from the PHA phasmid neurons (probably chemosensory) (White et al., 1986). It
has been postulated to be a sensory receptor itself on the basis of its
adult extension beyond the dorsorectal ganglion into the tail, although no
ultrastructural specialisation is seen there (ibid.). One might instead
speculate that its main function is developmental. If one considers that
it is just as important for a nervous system to be able to build itself as
to function correctly in the end, it makes sense that there be selective
pressure for neurons important in development even if they serve little or
no purpose in the final circuitry. Another candidate for such a cell in
the C. elegans nervous system is PVT.
This is a large cell demarcating the front of the preanal ganglion and
forming the most anterior link between the rectal epithelium and the
ventral ectoderm, which has no observed synaptic output and only a couple
of possible inputs. However no experiments have been performed to test the
suggestion that it too may be primarily involved in developmental
organisation. Of course one should beware of suggesting that every neuron
must have a major function; it is quite likely that there are also
redundant cells present that are not particularly important at any time.
The disarray seen in the ventral cord of the adult AVG reconstruction is very reminiscent of that seen in a reconstruction of a
mutant in the gene unc-3(e151) (figure 4.1, J G White, E Southgate and N Thomson,
unpublished results). In that case too there were several subbundles,
looking very similar to those of the AVG reconstruction; the majority of processes were on the right, including the
identifidable cluster of major motor interneurons which again retained
their internal organisation, but not their relative position in the bundle.
The defect appears to be restricted to the ventral cord since the nerve
ring was correctly organised according to several electron mircoscopic
criteria, but the phenotype of unc-3 mutants is much more severe than that after ablation of the AVG parent, and indeed in the reconstruction of the mutant it appeared that
some postembryonic motor neurons might be missing or not properly made.
There are two other uncoordinated genes for which mutants show relevant
defects. The DD and VD commissures can be
visualised by immunocytochemical staining with antibodies against the
neurotransmitter GABA (helping to confirm that the DD and VD classes are probably
GABAergic and inhibitory); they normally all grow to the right. However in
mutants for unc-71(e451) and unc-73(e936) a significant proportion of the commissures grow round the left side of the
animal (25% and 35% respectively; S McIntire, pers. Comm.). The ventral
cord is also seen to be disorganised, in that in some places in the cord
the VD and DD processes, which normally run so close together that they are inseparable
by light microscopy, are clearly separated. It would be interesting to see
what happens in early ventral cord development, particularly to AVG,
in all of these mutants.
The suggestion derived from the reconstructions of the adult AVG animal and the unc-3 mutant that left/right pairs of processes tend to stick together may be
significant. When the lumbar neuronal processes meet in the PAG at the
bottom of the lumbar commissures they "zip" together, each process in
contact with its homologue, except for PVQL/PVQR which remain apart (figure
3.9). Eventually PVQL/PVQR end up on separate sides of the cord, while the
others all stay together on the right side. This affinity of a process for
its opposite homologue provides a simple mechanism to ensure that processes
stay together. Then perhaps only a slight bias is needed to send the pair
to one side rather than the other. The experiments in which the parents of PVP cells were ablated reveal an underlying preference for the right side in at
least one case. When PVPR was removed PVQL crossed to the right side rather than grew along the left
side of the cord by itself, but when PVPL was removed PVQR still grew along the right side. It may be that the
presence of preexisting fibres on the right rather than the left was the
determining factor in this particular case, but after AVG,
the DD axons, DVA and DVC have grown out on the right side, which might prove sufficient to continue
to attract later arrivals.
To return to Meissner's suggestion the the primitive ventral nerve was
double, it may be worth discussing the advantages and disadvantages of a
fused cord over paired nerves. The obvious disadvantage of a single cord
like that of C. elegans is the loss of possible left/right control
over body movement. Although there are four bands of muscle in C.
elegans both ventral quadrants receive the same input from the right
hand ventral cord, as do both dorsal quadrants from the single dorsal cord.
Therefore the body of the animal moves only in the dorsal/ventral plane,
although the head can and does move freely in all directions. However there
are extra cross connecting motor neuron and interneuronal classes in the
head, and it is likely that in order to obtain reasonable left/right
coordination, something similar would be needed in the body. There is no
sign of this, even in vestigial form. On the other hand, if, as seems
likely, the putative primitive twin-nerved ancestor did not have the
capability for left/right body control (I have found no mention of any
nematode that does), then there is a strong case for bringing the motor
circuitry elements together in one nerve. First it allows an effective
halving of the number of motor neurons; with the system as it is in C.
elegans there is only one active motor neuron of each class at each
cross section of the body. Second it removes at source any loss of
synchrony between wave generation on the left and right sides of the body.
Third it provides back up in an extremely important part of the animal's
nervous system by having twofold redundancy of each motor circuitry driving
interneuron. However there is no obvious reason why the interneurons not
involved in the motor circuitry should join together or not, since they
serve no function in the cord but merely use it as a route from one end of
the cord to the other. Indeed this view is supported by the fact that a
minority of three apparently unrelated classes (AVK, PVP and PVQ)
are still bilateral in C. elegans.
In conclusion I would like to speculate that the primitive nematode ventral
cord was double and symmetric, and that the selection pressure for the
currently more common asymmetric cord came from the motor circuitry. It
appears that AVG plays a critical role in organising the left/right asymmetry of the motor
neurons. An important factor for the interneurons appears to be the mutual
affinity of left/right pairs (and of the motor circuitry interneuron
classes for each other, since they preserve their approximate relative
structure under perturbation by AVG parent ablation and unc-3 mutation). The interneuron pairs of groups may then tend to go to the
right side either directly or under the influence of AVG,
the motor neurons, or other previously determined processes, such as that
of DVA.
If this picture is correct then the fact that so many left/right pairs of
non-motor circuitry interneurons also join up and grow together on the
right would suggest that, even in situations like this where all the cells
are individually distinguishable, neural guidance may be often controlled
by non-specific factors that affect a large number of neurons.
5.3 Motor neuron outgrowth and formation of the dorsal cord
The preceding section described how the presence of AVG appears to help determine the side of the cord that the DD processes grow
along. A second question concerns how the DD processes growing
along the ventral cord know where to stop and send out their commissures.
Although they have short posterior processes, the main DD ventral cord
processes extend forward from the cell bodies, eventually making contact
with the next DD cell along. However
there is a certain amount of evidence to suggest that the determining
factor for DD ventral cord growth
may not be the next DD cell, but the
position of the next DB cell body. First the DD commissures always exit from next to DB cell bodies, even
when these are not immediately behind the next DD cell (e.g. DD3/DB4 in figure 3.4). Second there often seems to be some sort of recognition
event involving DD process tips
inserting themselves into DB cells at the time of
and soon after process outgrowth, particularly at the back of the cord
(figure 3.12). Third, in Ascaris, where distances are much greater, all the DD commissures exit opposite DB cell bodies together
with DB commissures, which are all on the right hand side behind the RBG (Johnson
and Stretton, 1987). VD and AS commissures also
grow out together in Ascaris (ibid.). Neighbouring VD and AS cells are sisters,
but there is no lineal relationship whatsoever between DB and DD cells (in C.
elegans, and presumably also in Ascaris, whose early lineage is
identical to that of C. elegans, Sulston et al., 1983).
Fourth, after DD3 and DD5 were removed by ablating their parent, DD4 did not extend to fill the whole space left by DD3,
but instead stopped and began sending out a commissure at an only very
slightly anterior position to normal (figure 4.3). This experiment does
not prove DB involvement,
however, because it remains possible that the normal growth length is
intrinsically determined, as appears to be the case with the postembryonic
touch cells AVM and PVM (Chalfie et al., 1983). A more conclusive, but unperformed,
experiment would be to remove a DB cell.
The next event after DD process outgrowth is
the growth of the motor neuron commissures. All the commissures grow out
synchronously and reach the dorsal midline at the same time, well before any
other longitudinal process has grown along the dorsal cord (RID will do so eventually). There is therefore a problem of recognising the
correct point at which to turn, and a subsequent problem of deciding the
direction in which to turn. Although adjacent to the basement membrane,
the commissural growth cones appear to grow on the surface of the
hypodermis, rather than the basement membrane (section 3.2). Similar
behaviour was inferred from experiments on early optic nerve outgrowth
(Krayanek and Goldberg, 1981). When the growth cones reach the dorsal
ridge they have been seen to insert finger-like extensions into the
hypodermis, indicating that some cell recognition event may have taken
place (figure 3.11). Therefore it seems that the best candidate for the
source of the required information is the dorsal hypodermal ridge itself,
and that the growth cone "tastes" the hypodermis as it advances, eventually
recognising the dorsal ridge.
The suggestion that there is a specific property of the dorsal hypodermal
ridge that is recognised, while simplifying the explanation of how the
dorsal cord is formed, creates problems of its own. The dorsal hypodermis
is a syncytium containing many nuclei and covering the dorsal side of the
animal from head to tail and from one lateral ridge to the other (the
lateral boundaries can be seen in the section in figure 1.1). The
commissure therefore grows on the surface of this syncytium for some time
before it recognises a specific part of it. In so doing it crosses the
path of some later longitudinal nerves, such as the ALM process, and the sublateral bundle (SAAD, SABD, SIBD, SMDD,
see figure 1.2). Hence it appears that some property of the membrane must
be localised to only that part of the cell surface covering the dorsal
ridge. The syncytium is formed in the embryo in a curious fashion by two
rows of cells passing between each other and then fusing. Mutations in two
genes, unc-83 and unc-84,
are known to affect this process (Sulston and Horvitz, 1982). Although
mutant L1 larvae move well, they have been seen in electron microscope
reconstructions to contain defects in the structure of the dorsal cord (J.
G. White, unpublished observation), which might be due to the failure in
the correct localisation of recognition components in the dorsal hypodermal
ridge.
Once the motor neurons have turned onto the dorsal cord, they seem to grow
out rapidly along it and, if they are DA or DB neurons, start
making neuromuscular junctions (D reconstruction, figure 3.6). It is only
at around this time or later that their dendrites grow out in the ventral
cord, so they start neuromuscular activity receiving organised synaptic
input. A system in which neurons generate synaptic activity before they
receive their controlling input would be expected to generate a lot of
random signals, but would allow the whole nervous system to be built
simultaneously instead of sequentially, starting with sensory neurons and
progressing along the processing pathway.
5.4 Discussion
Decussation of nerve processes, in which an entire group of cell processes
cross the midline, is a standard phenomenon in most animal nervous systems,
and a scaled down version of the same type of behaviour can be seen in C. elegans in the crossing over of processes from paired
interneurons in the preanal and retrovesicular ganglia. The PVP processes cross in the PAG (figure 3.8) and the RIF, RIG and SABV processes cross in the RVG (figure 3.10). Since the general
property of decussation appears to be functionally unnecessary, it may give
some insight into general constraints on developmental organisation.
It is very clear in C. elegans that there is no ultimate functional
advantage to be gained from the decussation. The crossovers are not used
to facilitate transfer of information from one side of the nervous system
to the other by receiving input on one side and having output on the other,
since in almost every case all the synapses and gap junctions observed in
the adult wild type reconstructions are on the parts of the processes
beyond the cross over point. The exception is that the RIF cells both make gap junctions to AVG on their cell bodies, but this also would not be logically different if the
cell body positions were reversed. It is not even the case that the
symmetrical body positions of the neurons involved are preserved into later
development; in fact the cell bodies in both the PAG and the RVG get
squashed into a single row as the muscles mature.
This situation is different from that in most vertebrate decussations, in
which the cells remain on the opposite side from their axonal termini, and
have some functionality on both sides. However, even there it is clear
that, considering the whole organism, there is more crossing over than is
necessary. An engineer would have the right side of the brain receive
information from, and control, the right side of the body. Some
communication between the two dies is certainly necessary, and this is seen
for example in the corpus callosum between the two hemispheres of the
cerebral cortex (and in the C. elegans nerve ring). However such
connections are inherently different from the general sensory and motor
decussations, for which the argument can still be made that they are
functionally necessary, and are more likely to reflect developmental than
functional constraints.
One common factor between the four miniature examples of decussation in the
PAG and RVC of C. elegans is that they are all between pairs of
neurons touching across the ventral midline. It might be suggested that
their mutual affinity causes their processes to grow towards the opposite
cell, and therefore cross over. However after either PVPL or PVPR was removed by ablating its parent the other stayed in position and still
sent its process across the midline and along the opposite side of the cord
as normal. In addition there are three pairs of cells in the ventral
ganglion in front of the excretory duct which are also adjacent across the
midline (AIA, SMBV and SAAD)
and none of them cross over. Instead the simplest unifying property of the
decussating pairs is regional: they comprise all the left/right pairs of
interneurons associated with the ventral hypodermal ridge between the
excretory duct and the anus. This, however, suggests neither a mechanism
nor a reason for the crossing over.
One possibility is that the crossing is ballistic: both processes are
attracted to some point or region on the midline and once they get there
they keep on growing in the same direction and thus cross over. Nerve
processes in vitro tend to grow in straight lines (Bray, 1979). The
attraction of the ballistic hypothesis is that it permits there to be no
intrinsic distinction between the two cells. The fact that all the
decussating pairs in the RVG cross in the same place supports the
hypothesis. Also the PVP crossing point in the PAG seems to be special, since the DVC process crosses from top left to bottom right in the same place, on its way
forward through the preanal ganglion. The change in position of DVC does not define the site, because removal of DVC by ablating its parent had no effect on the PVP processes and their crossover. Neither did removal of PVQL,
which normally contacts PVPR as soon as it crosses to the left and grows forward with it.
If we accept the ballistic hypothesis then it seems likely that PVT defines the site in the preanal ganglion, since the PVP processes cross between PVT and the processes of DVC and PVQ neurons, which are flattened out over the surface of DD6,
partially separating the PVP cells from DD6 (figure 3.8). Alternatively it may be that the site is defined by the DVC and PVQ processes in a redundant manner, so that removal of any one of them makes
no difference. The affinity of these three processes for the DD6 cell body is striking; they spread over its surface whereever it is
available, and when DVC was removed the PVQ processes spread further to mostly fill the gap (figure 4.7). Further
experimentation removing either PVT or DD6 might prove illuminating.
A variant of the ballistic hypothesis is that the initial directions of
outgrowth of the processes are both intrinsically towards the midline, and
so the processes simply cross over before turning forward. All the cells
involved migrate ventrally from lateral positions as the hypodermis closes
over the ventral surface of the embryo. It might be that the growth cones
start out continuing the direction of migration of the cell and thus cross
the ventral midline. This argument would apply equally well to the ventral
ganglion cell pairs that do not cross, and it is certainly not necessary
for an axon to leave a cell body in the same direction that the cell has
been migrating. For example the ALM cell bodies are seen migrating backward along the lateral hypodermis in the
C and D reconstructions, and in the E reconstruction they are sending axons
forward along the same path they have just followed but in the opposite
direction (figure 3.4). However, even if this does not provide a complete
explanation, it does suggest how intrinsic opposite polarities of the two
cells in each pair may be established.
5.5 Selective fasciculation
I have already suggested that AVG helps organise the ventral cord by providing a preferential path for
growth of, at the least, the DD axons. The wildtype
outgrowth of PVP and PVQ processes from the back of the cord, in which their tips always were found
very close together along the cord (section 3.5), suggested that there
might be some interaction involved. Therefore a series of ablation
experiments were performed to investigate PVP and PVQ outgrowth (section 4.3).
PVP and PVQ processes grow on both sides of the ventral cord. The left hand cord
contains only three processes at hatching, PVPR, PVQL, and AVKR (plus RMEV at the front, see fig. 1.3). The normal sequence of events is that PVPR and PVQL grow forward together, and AVKR was only seen to be growing back after they had reached the front. It
appears that PVPR is needed for the other two to grow on the left side, because when it is
removed no processes are seen in either the embryonic or adult left hand
cords (figure 4.4). If PVQL is removed then PVPR still grows forward along the cord by itself. Therefore, although PVQR is
not a unique pioneer in normal development because the PVQL growing tip is
parallel with its own, it does appear to have a primary role in
establishing the left hand cord. When PVPR is removed the PVQL process
still grows forward along the cord, but on the right side rather than the
left, and apparently somewhat delayed compared to PVQR and PVPL which normally grow on the right. In this case therefore the ability to
grow and the basic directionality of growth are preserved, although the
actual path taken was altered, as when AVG was removed. This corresponds to what is seen when guideposts are removed
in the insect PNS (Berlot and Goodman, 1984), or motor neurons in the
chick embryo (Landmesser and Honig, 1986). It is not known whether the AVKR process also extended along the right hand cord in the absence of PVPR and PVQL on the left side.
These results are not symmetrically reproducible on the other side of the
ventral cord, since PVQR still grows forward along the right side in the
absence of PVPL.
However, as discussed above, the cord is not symmetrical. While PVQR and PVQL are the first processes to grow along the left side of the cord, there
are other preexisting processes on the right at the time when PVQR grows
forward (AVG and DD axons) which might provide some degree of non-specific affinity that
assisted PVQR in growing along the right side. This could in principle be
tested by removing PVPL, AVG and DD6.
Although the removal of PVQL had no effect on the outgrowth of PVPR along the left hand ventral cord, it did appear to affect the growth of
other processes down the lumbar commissure from the left lumbar ganglion to
the preanal ganglion (see figure 1.3 for a schematic plan of the normal
situation). In neither of the reconstructed embryos in which PVQL had been
removed did any of the left lumbar processes grow down lumbar commissure,
although they had done so on the right side. As well as containing
processes descending from the lumbar ganglion, the lumbar commissures
contain a DA motor neuron process
ascending from the preanal ganglion. This was present in both the PVQL reconstructions.
These results suggest that there is a specific need for PVQL in order for
the other lumbar ganglion cells to grow correctly in the right direction.
Similar behaviour is seen in the developing grasshopper CNS, where in
several cases it has been shown that an identified neuronal growth cone
normally fasciculates with a specific preexisting fascicle, in the absence
of which it fails to grow in any organised fashion (Raper et al.,
1984, Bastiani et al., 1986, duLac et al., 1986). In one
case it was shown that a specific subset of the processes in the
preexisting fascicle is required (Raper et al., 1984). This
corresponds to the observation that the DA process in the
lumbar commissure is not sufficient to promote growth of other processes
down the commissure.
If PVQL provides guidance for the left lumbar processes by some process of
selective fasciculation, then this fasciculation does not last for long.
When processes from the two lumbar commissures meet in the preanal ganglion
all the cell types other than PVQ immediately form contact with their bilateral homologues, "zipping up" with
each other (figure 3.8). The other left lumbar processes then leave PVQL to
join their right hand homologues and PVQR on the right hand side.
Therefore it seems that there is a hierarchy of affinities that applies the
left lumbar processes other than PVQL; first they follow, and in fact
require, PVQL, then they leave PVQL in order to join their right hand
homologues.
These observations all fit the "labelled pathways" hypothesis (Ghysen and
Jansen, 1979, Goodman et al., 1982), that growth cones are
programmed to recognise a sequence of surface labels on fascicles, possibly
in some adhesive hierarchy, and that this determines their path through the
developing nervous system. The situation when the left and right lumbar
processes meet is somewhat novel, in that then two equivalent sets of
processes fasciculate together, and must decide which of the two PVQ neurons to follow. There is no good clue as to what determines this
(discussed earlier in the section on cord asymmetry.
The observations about lumbar commissure formation contrast with those made
in the ventral cord that, even if normal cues are missing, processes tend
to keep on growing in the correct direction. A plausible explanation of
this difference is tat there is a non specific property of the ventral cord
which permits or promotes neuron growth along it. Apart from the presence
of other processes, at the relevant time there is a continuous line of
motor neuron cell bodies along the ventral midline, which may act as
general guideposts in the same way as neuronal cell bodies that have been
proposed to facilitate neuron outgrowth in the insect PNS (Bentley and
Keshishian, 1982).
There are a number of uncoordinated mutants that are known to be defective
in outgrowth of processes from the lumbar ganglion cells, on the basis of
fluorescent staining of the PHA and PHB phasmid sensory neurons by direct uptake of fluorescein isothiocyanate
(Hedgecock et al., 1985). Mutants in unc-33, unc-44 and unc-76 all show the same phenotype. Rather than growing forward into the preanal
ganglion the phasmid axons stop abruptly where they meet at the bottom of
the lumbar commissures, often with swollen endings. This is at the point
where the resorting of the fibres takes place, with the majority of the
left lumbar processes leaving PVQL to grow forward with their contralateral
homologues. The fact that there are several genes with both this phenotype
and also defects in movement is interesting in relation to a suggestion
made earlier (in the discussion of ventral cord asymmetry). This proposed
that the mutual affinity of ventral cord bilateral homologues may be a
basic general mechanism whose biological purpose is to bring together the
motor circuitry interneurons, and which affects other neurons incidentally.
A prediction of this hypothesis would be that the anterior motor circuitry
interneurons would also be affected by the mutations. In mutants for unc-6 (referred to as unc-106 in Hedgecock et al.), the PHA and PHB axons normally fail to grow down the lumbar commissures, but instead wander
forward along the lateral hypodermis. This is reminiscent of the defect
seen in the left lumbar commissure when PVQL was removed. However the
defect in unc-6 mutants is more general than that following PVQL removal, since axons from
the postembryonic PVD neurons on the lateral hypodermis also fail to reach the ventral cord, and
motor neuron commissures are also disrupted (S McIntire, personal
communication).
5.6 Conclusion
In the introduction to this part of the dissertation it was proposed that a
number of different mechanisms could be used to influence neuronal
guidance, often concurrently, and a list of possible types and sources of
influence was provided. The behaviour of outgrowing neurites in both
normal and experimental C. elegans embryos that has been described
here has suggested new examples of several different types of influence.
The formation of the dorsal cord could be explained by the presence of a
preexisting preferred pathway along the dorsal hypodermal ridge. This would
essentially be an epidermal blueprint, as proposed by Singer et al. (1979). DD growth along the
ventral cord may be limited by some inhibitory effect of DB cells, although from
the observations that are available the inhibition seems more likely to be
caused by selective recognition accompanied by membrane insertion than by
the retraction of growth cones as seen by Kampfhammer et al. (1986)
in vitro. The decussation of processes in the preanal and retrovesicular
ganglia may be due to the tendency of growth cones to grow in straight
lines, as discussed by Bray (1979). In the lumbar commissures and the
determination of which processes grow along the left and right nerve cords
there appear to be several examples of selective fasciculation, similar to
that proposed in the labelled pathways hypothesis (Ghysen and Jansen,
1979). There also appeared to be a general directionally or premissive
property of the ventral cord region that meant that, even when specific
cues were removed, processes still grew out along the cord.
All these proposed interactions fall broadly into some class of interaction
that has been suggested previously. Further experiments of the same type
as described here, some of which I have mentioned in the discussion, could
be carried out to define more precisely the characteristics of particular
interactions. The other possible approach to further work is to use the
existing picture as a basis for an investigation of the genetic factors
controlling neural outgrowth, eventually uncovering the critical molecular
mechanisms involved using molecular genetic techniques (Greenwald, 1985).
In this discussion I have mentioned a number of mutants that affect neural
guidance in the ventral nervous system, in some cases in ways that are
partially interpretable in terms of the mechanisms proposed here. The
genetic approach is discussed further in the final conclusion after Part
II.
|