
|
|
|
NERVOUS SYSTEM IN THE EMBRYO - NERVE RING DEVELOPMENT
 Click pictures for new window with figure and legend, click again for high resolution image Click pictures for new window with figure and legend, click again for high resolution image
1 Overview
The C. elegans nerve ring (NR), along with the ganglia of the head, is considered to be worm's brain. It is a tight axon bundle made of processes from over half of the animal's neurons and is the most synapse-rich part of the nervous system. These synapses made en passant between neurons within the NR govern most of the animal's behaviors. In postembronic animals neuron processes are seen to enter the NR from distinct ventral, lateral, subdorsal and dorsal routes (EmbryoNRDevFIG 1 and EmbryoNRDevTABLE 1). Neuron processes follow either direct paths to enter the NR or travel through indirect trajectories from their cell bodies, i.e. via commissures. Most neurons in the lateral ganglia reach the NR from the ventral side via the amphid commissures while a subset of them take a more direct lateral route to enter the NR (see Hermaphrodite Chapter-Nervous System General Description and Ganglia and Longitudinal Tracts). In the early stages, head muscle cells migrate from more central positions around the NR to the periphery of the embryo and separate the lateral ganglia from the ventral as they do so (see Hermaphrodite Chapter-Muscle System Introduction). Commissural axons must therefore travel under the muscle to reach the ventral ganglion. Entry to the NR from the the ventral route is the earliest, while lateral, subdorsal and dorsal routes are established later (see below). The initiation and positioning of the presumptive NR, as well as the establishment of these tracts, lay down the pattern for later interactions between neurons within the final organization of the worm's largest neuropil.
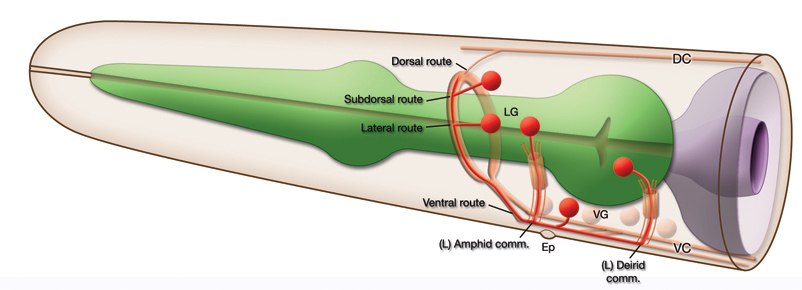 EmbryoNRDevFIG 1: Routes of Entry to the Nerve Ring (shown in postembryonic head). Most neuron processes enter the NR from the ventral side either directly or indirectly. However, a subset of the head neurons have entry to the NR from three other routes; dorsal, subdorsal and lateral. Note the neurons that are anterior to the nerve ring and have posterior processes that enter the NR are not indicated in this picture.
| Lateral route entry |
Subdorsal route entry |
Dorsal route entry |
| AVA |
RIA |
ALA |
| ADE |
RIV |
CEPD |
| ADL |
SAAV |
CEMD (male) |
| AIN |
SMDV |
RID |
| AVD |
|
|
| AVE |
|
|
| AVH |
|
|
| AVJ |
|
|
| RMDV |
|
|
| RMDL/R |
|
|
EmbryoNRDevTABLE 1: Neurons that enter the nerve ring from the dorsal, subdorsal and lateral routes. Note, neurons that are anterior to the NR are not listed.
|
2 Placement and Bundling of the Nerve Ring
In wild type C. elegans, after elongation, the NR is positioned around the center of the isthmus of the pharynx (EmbryoNRDevFIG 2). CWN-2, the C. elegans Wnt5 homolog, and SIA and SIB neurons play an essential role in maintaining the placement of the NR correctly in this position, likely during the elongation stage (Kennerdell et al., 2009). CWN-2 is required after 1.5-fold stage of embryogenesis for correct positioning, and functions through the Wnt receptor CAM-1. CWN-2 is a ligand for CAM-1 (Ror receptor) and CAM-1 expression in SIA and SIB neurons governs this function, although the CWN-2 expression site is not as important. MIG-1 may be acting as a co-receptor with CAM. When this pathway is perturbed the NR is displaced towards the anterior along with neuronal cell bodies, muscle arms and the excretory pore (EmbryoNRDevFIG 2) (Kennerdell et al., 2009). In cwn-2-mutant animals SIA and SIB process guidance is also defective such that these sublateral axons often fail to exit the nerve ring or exit in the wrong place (Kennerdell et al., 2009). The anterior misplacement of the NR is also observed when SAX-3 axon guidance receptor is mutated. Also, in these mutants the NR is no longer an intact single bundle but instead defasciculates into several bundles (Zallen et al.,1998 and Hao et al., 2001).
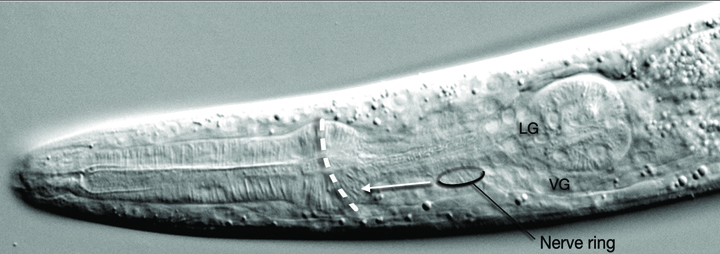
EmbryoNRDevFIG 2: Positioning of the Nerve Ring. In wild type animals the NR is placed around the isthmus of the pharynx. However, in CWN-2/CAM-1 pathway mutants, both the NR and the neurons are misplaced towards the anterior and the ring is placed around the anterior bulb of the pharynx (indicated by white dashed line). Differential interference contrast (DIC) image of the head of a wild type young adult, left lateral view. Magnification, 600x.
|
3 Formation of the Nerve Ring
In the embryo, the NR assembly initiates between bean and comma stages and continues up until the end of embryogenesis, The NR is visible around the embryonic pharynx by the 1.5-fold stage (EmbryoNRDevFIG 3) (Rapti et al., 2017) (for stages of embryonic development see Hermaphrodite Chapter-Introduction to C. elegans Anatomy and IntroFIG 7). Its assembly is hierarchical; processes from five bilateral sublateral commissure neurons (SIAV, SIAD, SIBV, SIBD, SMDD) and the CEPshV glia merge to form a pioneer process bundle to enter and initiate the presumptive NR. The growth of follower processes occurs in waves subsequently. At the onset of NR formation, sublateral neuron processes fasciculate and navigate towards the CEPshV, they then coalesce with the dorsally directed process of CEPshV, which likely defines a turning point for the sublateral process bundle. Early neuronal axons and glia processes forming this pioneer nerve-fascicle of 10 or fewer processes then cooperatively direct follower axon entry to the newly born NR. Supporting their pivotal role in patterning the developing NR, SIA and SIB processes follow a complex path around the NR in the adult, occupy positions near the middle of the NR neuropil and have few synaptic connections. In CEPsh-ablated animals the pioneer sublateral process bundle still enters the presumptive NR, however, it is defasciculated and extends anteriorly in an aberrant fashion compared to the fascicle in nonCEPsh-ablated animals. Ablation of CEPshD or CEPshV glia correlates with AIY, AWB and AWC axon entry defects towards the NR. SIA and SIB ablation also leads to defects in AIY and amphid neuron process entry to the NR, although CEPsh glia process growth and wrapping of the NR are not affected. KPC-1 (a Kex2/subtilisin-like proprotein convertase) and CHIN-1 (a GTPase activating protein) function in CEPsh glia to guide the sublateral pioneers during the earliest NR assembly, and function in both the sublaterals and CEPsh glia for the navigation of the follower axons (Rapti et al., 2017). An axon guidance molecule, UNC-6, is produced by CEPshV cells and when mutated leads to defects in ventral NR processes and synapses (Wadsworth et al., 1996; Colon-Ramos et al., 2007, Yoshimura et al., 2008). For pioneer-process guidance, UNC-6/Netrin expression in CEPsh glia is essential and for follower-process guidance, MAB-20/Semaphorin and FMI-1/CELSR expression in both the glia and the sublaterals are essential (Rapti et al., 2017). The majority of follower amphid axons first start to extend from their cell bodies around 350 min (between bean and comma stages) after first cell division and have already completed their path to the nerve ring around 430 min after first cell division (around about 1.75-fold stage, 8 amphid commissure axons are seen entering the NR). At the 1.75-stage, the neuron processes that take the lateral route to the NR and the two processes of ALA which enter the NR via the dorsal route are only beginning to grow out. The latter two route may be blocked until muscle cell migration to the periphery opens up access by these processes to the NR (Norris C. and Hall D.H. unpublished observations 1997) (EmbryoNRDevTABLE 2) (EmbryoNRDevFIG 4). As the amphid commissure is forming from the lateral ganglion neurons extending towards the ventral, the amphid axons may utilize a large neuron (possibly SMBD or SMDD) situated between muscle and hypodermis as substrate for growth (unpublished observations, C. Norris and D.H. Hall, 1997).
| Neuron |
Adult Location |
Type |
Axon outgrowth starts |
Axon Enters Presumptive Nerve Ring |
| Axons entering the nerve ring from the ventral side (except BAG) |
| SIAV |
Ventral ganglion |
ventral sublateral cord, amphid-commissural |
Bean stage |
Comma stage |
| SIAD |
Ventral ganglion |
dorsal sublateral cord, non-commissural |
Bean stage |
Comma stage |
| SIBV |
Ventral ganglion |
ventral sublateral cord, amphid-commissural |
Bean stage |
Comma stage |
| SIBD |
Lateral ganglion |
dorsal sublateral cord, amphid-commissural |
Bean stage |
Comma stage |
| SMDD |
Ventral ganglion |
dorsal sublateral cord, non-commissural |
Bean stage |
Comma stage |
| AWC |
Lateral ganglion |
amphid-commissural |
Late bean stage |
1.5-fold stage |
| AIY |
Ventral ganglion |
non-commissural |
Comma stage |
1.5-fold stage |
| BAG |
Anterior ganglion |
non-commissural |
Comma stage |
1.5-fold stage |
| AFD |
Lateral ganglion |
amphid-commissural |
Comma stage |
2-fold stage |
| ADA |
Non-ganglionic, posterior lateral in the head |
deirid-commissural |
Late comma stage |
After 2-fold stage |
| BDU |
Anterior body |
deirid-commissural |
Late comma stage |
After 2-fold stage |
| AUA |
Lateral ganglion |
amphid-commissural |
1.5-fold stage |
2-fold stage |
| ASE |
Lateral ganglion |
amphid-commissural |
Late 2-fold stage |
After 2-fold stage |
EmbryoNRDevTABLE 2: Timeline of axon outgrowth and entry into the nerve ring by neuron processes. CEPsh glia and sublateral neuron processes initiate the NR which is subsequently populated by non-commissural, amphid-commissural and deirid-commissural neuron processes in an orderly fashion (Rapti et al., 2017). Comma stage is at about 430 min after fertilization (i.e. ~390 min after the first cell division), 1.5-fold stage is at about 460 min after fertilization (~420 min after the first cell division) and two-fold stage is at about 490 min after fertilization (~450 min after the first cell cleavage) at 20-22oC (Rapti et al., 2017).
EmbryoNRDevFIG 3: The initiation and formation of the nerve ring. A-D. Fluorescent images of embryos corresponding to the stages shown in the right hand column. Scale bar: 10 µm. E-G. Graphic images of nerve ring development in different stage embryos. Cell colors follow the WA color code. A-C. Pceh-17::GFP marker; SIA, SIB, DA5, DA8 pseudocolored red, Pttx-3::mCherry marker; SMDD pseudocolored yellow. D. Phlh-16::GFP marker; SMDD pseudocolored red, AWC pseudocolored magenta. A&E. Comma stage embryo. Sublateral commissure neuron processes navigate anteriorly towards CEPshV and bundle with the dorsally-directed processes of these glia to position and initiate the nascent NR (among the sublaterals, SIA and SIB neuron processes grow slightly earlier than SMD processes and may be sufficient for this function) C&D, F. 1.5-fold embryo. CEPsh and sublateral neuron axons cooperatively drive the follower processes (e.g. AIY, amphids) to enter the NR. White arrowhead, AWC amphid process which follows sublaterals into the nascent NR; white thin arrows, AWC dendrite stretching posterogradely from the lip (Heiman &Shaham, 2009), white arrows, opposite side sublateral axons that reach each other at the NR midline by this stage G. 1.75-fold embryo. In the older embryo muscles have migrated and separated into dorsal and ventral quadrants opening direct access to the NR from the lateral ganglia. The time of muscle separation correlates with the extension of the first process (white arrow) in the lateral route (likely that of AVA neuron) (C. Norris and D.H. Hall, unpublished observations, 1997). While the mesoglial GLRs line the interior of the NR in adults, in the embryo, they are aligned around the posterior border of the NR while RME neurons surround the peripheral border of the NR (C. Norris and D.H. Hall, unpublished observations).
|
EmbryoNRDevFIG 4: Development of the amphid commissure and the lateral route to the nerve ring (NR). A-C. TEM images (cross sections) of a 350-360 min embryo (after first cell division, approximately between bean and comma stages) showing the nascent commissure fibers traveling between the head muscle (Head mu) and the SIBD neuron that has lodged itself between the muscle and the hypodermis (Hyp). Anterior and posterior to these nascent commissure fibers, head muscles are attached to the hypodermis. (Image source: [Hall archive] N611_sections386, 431, 522.) Inset. Schematic representation of the amphid commissure development. At this stage muscle cells (Mu) that surround the pharynx primordium may be preventing a more direct, i.e. lateral, route to the NR (indicated with crosses) for the processes of the lateral ganglia neurons that are trying to reach the NR. Commissural axons must therefore travel under the muscle to reach the ventral ganglion. SIBD neurons that are positioned between the muscle and the hypodermis at this stage may be providing a bridge between the lateral and ventral ganglia for the early commissural axons. EC, excretory cell; LG, lateral ganglion; VG, ventral ganglion. D&E. Cross sections of TEM images of comma stage when the NR pioneer bundles (marked by orange dotted lines) are seen on each side and 1.5-fold embryos when the NR is visible around the pharynx primordium, are shown. A, anterior; P, posterior; D, dorsal; V, ventral (Image source: Shaham Lab.) F. Schematic representation of the NR development in 430 min embryo (after first cell division, approximately between 1.5-fold and 2-fold stages). Muscle cells have migrated to their final positions next to hypodermis, leaving a trailing process behind that remains attached close to the pharynx. Processes from the remaining lateral ganglia neurons can now reach the NR directly from a lateral route rather than having to travel ventrally first. The pioneering neuron to grow a lateral process into the NR is thought to be AVA. Ventrally, RIH is positioned to act as a guidepost for processes growing into the NR (C. Norris, D. H. Hall, E. Hedgecock, unpublished observations). |
EmbryoNRDevTABLE 3: Development of CEPsh glia and the NR pioneers in the embryo. The movies span about 360 min and end around 1.5-fold stage, just as twitching starts in the underlying embryo. Note SIAD and SIBD are not shown in the movies. Cells do not follow the WA color code; top panel shows the color code used in the movies. 0 min is fertilization. Click on the movies for higher resolution renditions (by A. Santella & Z. Bao).
4 References
Colon-Ramos, D.A., Margeta, M.A. and Shen, K. 2007. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318: 103-106. doi: 10.1126/science.1143762. Article
Hao, J.C., Yu, T.W., Fujisawa, K., Culotti, J.G., Gengyo-Ando, K., Mitani, S., Moulder, G., Barstead, R., Tessier-Lavigne, M. and Bargmann, C.I. 2001. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 32: 25-38. doi: 10.1016/S0896-6273(01)00448-2. Article
Heiman, M. G. and Shaham, S. 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137: 344-355. doi: 10.1016/j.cell.2009.01.057. Article
Kennerdell, J.R., Fetter, R.D. and Bargmann, C.I. 2009. Wnt-Ror signaling to SIA and SIB neuron directs anterior axon guidance and nerve ring placement in C. elegans. Dev. 136: 3801-3810. doi: 10.1242/dev.038109. Article
Rapti, G., Li, C., Shan, A. and Shaham, S. 2017. Glia initiate brain assembly through noncanonical Chimaerin–Furin axon guidance in C. elegans. Nat. Neurosci. 20: 1350-1360. doi:10.1038/nn.4630. Article
Wadsworth, W.G., Bhatt, H. and Hedgecock, E.M. 1996. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16: 35-46. doi: 10.1016/S0896-6273(00)80021-5. Article
Yoshimura, S., Murray, J.I., Lu, Y., Waterston, R.H. and Shaham, S. 2008. mls-2 and vab-3 control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Dev. 135: 2263-2275. doi: 10.1242/dev.019547. Article
Zallen, J.A., Yi, B.A. and Bargmann, C.I. 1998. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 92: 217-227. doi: 10.1016/S0092-8674(00)80916-2. Article
|

This chapter should be cited as: Altun, Z.F. 2017. Nervous system in the embryo, development of the nerve ring. In WormAtlas. doi to be announced
Last revision: Oct 26, 2017 |

|
|
|
|