|
The buccal capsule of Caenorhabditis elegans (Nematoda: Rhabditoidea): an ultrastructural study
K.A. Wright1 and J.N. Thomson2
1Zoology Department, University of Toronto, Ontario, Canada
2M.R.C. Laboratory of Molecular Biology, Hills Road, Cambridge, CB2 2QH, England
Canadian Journal of Zoology (1981) 59: 1952-1961
doi: 10.1139/z81-266
(Received April 16, 1981)
Abstract - Introduction -
Material & Methods -
Results -
Discussion -
Acknowledgments
- References
Abstract
The buccal capsule of the free-living nematode Caenorhabditis elegans has been analysed by serial section electron microscopy. Whereas the regions classically identified in the rhabditid buccal capsule can be distinguished, the cuticle lining does not constitute separate cuticular plates, but rather, structural-functional differentiations within a cuticle continuous with that of the esophagus. Only the lip region (cheilostom) is lined by body wall cuticle. The prostom cuticle is underlain by two rings of syncytial arcade cytoplasm connected to nine cell bodies. The mesostom cuticle is underlain by the nonmuscular epithelial cells of the esophagus, whereas the cuticle of the metastom and telostom is underlain by esophageal muscle cells m1 and m2. During moulting, buccal cuticle is produced later than body cuticle and its formation is characterized by accumulation of dense granules in both arcade and esophageal cytoplasm. It is concluded that the buccal capsule should be considered as "astomatous" in the terminology of K. A. Wright.
Introduction
The buccal capsule of nematodes functions in the initial uptake of food into the alimentary tract. As such it shows many modifications in form dependent on the nature of food and feeding mechanism. Hence, buccal structures have been used extensively in taxonomic and phylogenetic considerations. The buccal capsule of rhabditoid nematodes has received considerable attention and a complex terminology, initially attributable to Steiner (1933), has developed to describe its form in various subgroups of the order Rhabditida. Most commonly the buccal capsule has been considered to include regions named the cheilostom, prostom, mesostom, metastom, and telostom. Attempts have also been made to extend this terminology (by implication, through homology) to other major nematode groups (e.g. De Coninck 1965; Coomans 1963; Ritter 1965), although pitfalls have been recognised (Coomans et al. 1978).
Recent anatomical studies using electron microscopy have noted marked differences between cuticle of the body wall and that of the nematode's esophagus (Dick and Wright 1973; Wright 1976). Although the buccal capsule of some groups may consist of only slight modification of anterior musculature of the esophagus which makes contact with the body wall directly at the oral opening, in others the body wall cuticle and underlying cells may infold into the buccal capsule to make contact with the esophagus some distance internally. With the extensive development of serial-section electron microscopy of Caenorhabditis elegans, and experimental studies of its development, it has been possible to analyse in more detail the structure of the buccal capsule and its formation during moulting. A previous study of the cellular composition of this nematode's esophagus (Albertson and Thomson 1976) formed a basis for part of this study.
Materials & Methods
The animals studied were from the Caenorhabditis elegans (N2) strain maintained at the Laboratory of Molecular Biology, Cambridge University. Cells of the head were traced through one series of more than 800 sections of a normal (N2) hermaphrodite animal. The general cell arrangement was confirmed in a second less complete series of a normal hermaphrodite, and a series of longitudinal sections was cut of another. These worms were fixed only in 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h. Worms in various stages of moulting were selected according to criteria established by Singh and Sulston (1978). These were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 2 h and postfixed 1 h in 1% OsO4. They were oriented in agar, dehydrated, and embedded in Araldite epoxy. Sections were stained with uranyl acetate and lead citrate.
Living animals were examined by differential interference microscopy in bacterial culture preparations as described by Sulston (1976) and photographed with electronic flash.
Results
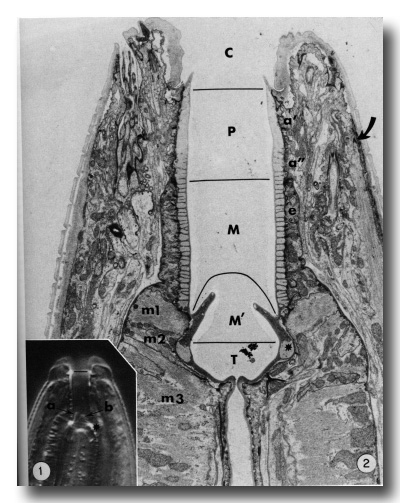
The general form of the buccal capsule is well seen in living animals studied by interference microscopy (Fig. 1). The most obvious unit is a subtriangular cylindroid recessed slightly from the oral opening. The basal half of the cylindroid is surrounded by a thin collar-like extension of the esophagus, and its base is embedded directly in the anterior esophagus. In the base, three triangular flaps occur. The triradiate esophageal lumen continues from the base of the buccal capsule and the large tubular radii of the esophagus' procorpus lumen thus open to the base of the buccal capsule. The dorsal esophageal gland opens into the esophageal lumen just where the lumen begins to assume a triradiate form.
 Figures 1 and 2.
Figures 1 and 2.
Figure 1.
Interference photomicrograph of the buccal capsule of a living adult Caenorhabditis elegans.
The dorsal metastomal flap (a) is seen in edge view, and a subventral flap (b) is seen in face view. The cheilostom-prostom limit is noted by the short line. Asterisk notes prominent refractile cuticle of the telostom. X2000.
Figure 2. Longitudinal section through the buccal capsule of an adult. The classically identified regions of the buccal capsule are the cheilostom (C), prostom (P), mesostom (M), metastom (M'), and telostom (T). Prostom cuticle is underlain by anterior (a') and posterior (a") arcade tissue. Mesostom cuticle is underlain by the anterior epithelial cells of the esophagus (e), and muscle cells m1 and m2 underly cuticle of the metastom and telostom respectively. Muscle cell m3 comprises the procorpus of the esophagus. Asterisk identifies the telostom cuticle that is refractile in light microscopy. The anterior tip of a somatic muscle is noted on the right (arrow), x 11 000.
Electron micrographs clearly show that body wall cuticle lines the inner surface of the lips and makes a discrete contact with the cuticle that forms the prominent cylindroid (Figs. 2 and 3).
 Figures 3 and 4.
Figures 3 and 4.
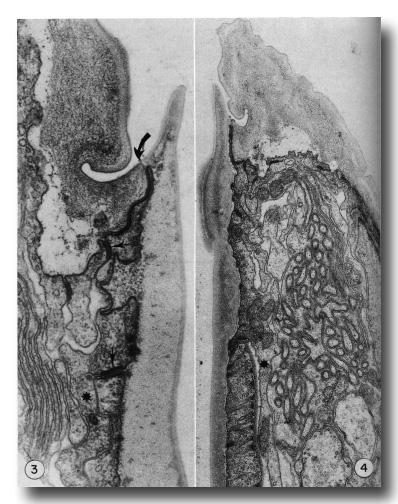
Figure 3. Detail of the contact (large arrow) of body wall (cheilostom) cuticle and esophagus (prostom) cuticle. Note the contact of anterior arcade tissue with body wall hypodermis and the posterior arcade tissue (small arrows). Asterisk notes a posteriorly directed process of the anterior arcade tissue. Note also the infolded groove in the cheilostom cuticle, x 58 000.
Figure 4. Section through the dense "corner" of the buccal cuticular cylindroid. Arcade tissue appears to be more strongly attached to cuticle here, with many cytoplasmic filaments along the inner membrane. Asterisk notes a posteriorly directed process of the posterior arcade tissue lying outside the esophagus, x 26 500.
Cuticle of the lips is underlain by thin processes of hypodermal cytoplasm. At the contact with the cuticular cylindroid the hypodermis forms a continuous junctional complex (belt junction) with a ring of cytoplasm that encircles the buccal cuticle. This latter cytoplasm extends only 1.5 micrometer posteriorly, where continuous junctions join it to a second ring of cytoplasm that similarly encloses the buccal cuticle and about 2 micrometer posteriorly contacts the esophagus proper (Figs. 3, 4, and 5). The two units of cytoplasm with their associated cell processes will be referred to as the anterior and posterior arcade tissue. In the intermoult stage, arcade cytoplasm contains few mitochondria and scanty endoplasmic reticulum or other organelles, although there are numerous circularly oriented filaments in the cytoplasm. As these are prominent in worms fixed only in osmium it is likely that they are a class of tonofilaments (intermediate filaments) rather than actin. Many filaments appear to insert obliquely into the junctional complexes between cells.
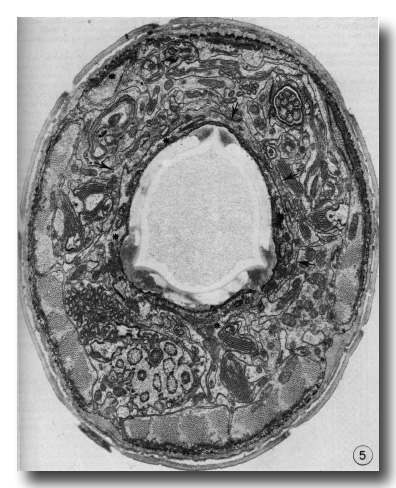 Figure 5. Cross section through the buccal cuticle just above the level of the prostom-mesostom change showing the shape of the cuticle with three dense "corners." At this level the posterior arcade cytoplasm (asterisks) entirely surrounds the cuticle while some of the anterior arcade cytoplasm (arrows) lies just outside it, x 15 000.
Figure 5. Cross section through the buccal cuticle just above the level of the prostom-mesostom change showing the shape of the cuticle with three dense "corners." At this level the posterior arcade cytoplasm (asterisks) entirely surrounds the cuticle while some of the anterior arcade cytoplasm (arrows) lies just outside it, x 15 000.
The anterior arcade cytoplasm gives rise initially to four posteriorly directed cell processes, three in the subdorsal fields and one in the ventral. The processes lie outside the esophagus (Fig. 4). One of the subdorsal processes soon ends and the remaining processes move laterally to lie, one in the dorsal aspect of each lateral hypodermal chord and one in the ventral chord. Cell bodies of these processes occur in the inner aspects of these chords (not in contact with cuticle) about 50 micrometer from the head where the tubular radii of the esophageal lumen end. Cell processes arising from the larger posterior arcade cytoplasm originate rather symmetrically as a dorsal and a ventral process, and as four processes one on either side of the lateral chords. As they progress posteriorly, the dorsal and right dorsolateral processes fuse, but again separate and connect ultimately to two cell bodies. The right ventrolateral process terminates at the level of the anterior procorpus. Considerably posterior to this the ventral process bifurcates. Thus, six cell processes were ultimately traced to cell bodies, one dorsally, one just above the right lateral hypodermal chord, one just above and one below the left lateral hypodermal chord, and two (from the bifurcated cell process) at either margin of the ventral hypodermal chord. These cell bodies lie just behind those of the anterior arcade tissue (see diagram, Fig. 6).
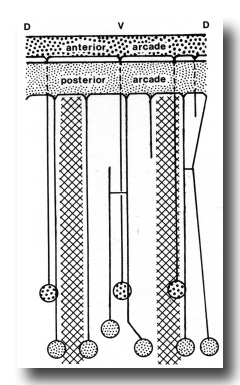 Figure 6. Diagram of arrangement of syncytial arcade tissues, with connected cell bodies. Syncytia have been opened along the dorsal line. Cross hatched areas indicate lateral hypodermal chords.
Figure 6. Diagram of arrangement of syncytial arcade tissues, with connected cell bodies. Syncytia have been opened along the dorsal line. Cross hatched areas indicate lateral hypodermal chords.
The remainder of the cuticle of the buccal capsule is enclosed by cells of the esophagus. The entire esophagus is sheathed by a conspicuous basal lamina. The nine most anterior epithelial cells of the esophagus (described by Albertson and Thomson 1976) form the esophageal collar that surrounds the basal part of the cylindroid cuticle while the first two muscle cells (m1 and m2 of Albertson and Thomson (1976)) underly the buccal cuticle where it changes shape to the triradiate lumen of the procorpus (Fig. 2).
Although the cuticle of the buccal capsule is continuous from the point where it makes contact with body wall cuticle throughout the entire lining of the esophagus, it displays internal differentiations that can be readily equated with the regions of the buccal capsule recognised in taxonomic studies (Fig. 2). Thus, the cheilostom is that region enclosed by the lips and lined by body cuticle. The prostom and mesostom compose the subtriangular cylindroid cuticle. The presence of densities that traverse the cuticle in the region enclosed by the esophagus' epithelial cells allows distinction of the mesostom from prostom. However, throughout the prostom and mesostom the cuticle at the subdorsal and ventral apices of the "triangle" is also dense (Figs. 4 and 5). The anterior rim of the prostom cuticle projects inward as a slight flange into the anterior lumen of the buccal cavity. The metastome is that region from which the three triangular flaps arise. The cuticle is uniformly dense and is underlain by the m1 muscle cell. Just below this the cuticle has a less dense region in dorsal and subventral sectors that is responsible for the prominent retractile spots noted by light microscopy. This area and the remaining dense cuticle underlain by the m2 muscle cells can be recognised as the telostom.
Densities in the mesostomal cuticle are coincident with rows of small hemidesmosomes and tonofilament bundles that traverse the esophagus epithelial cells. These cells are apparently tightly adherent to the cuticle, whereas arcade cytoplasm may adhere mainly only along the dense apices of the "triangular" cuticle.
Myofilaments of the m1 muscle cell, which forms a complete ring around the front of the esophagus, converge from the outer membrane to the inner cell membrane at the anterior base of the metastomal flaps. Similarly, myofilaments of m2 muscle cells converge on the lumenal membrane just below the metastomal flaps. The dorsal esophageal gland duct opens into the buccal capsule by penetrating through the dorsal m2 cell. Cuticle of the esophagus lumen projects into the end of the gland cell forming a fluted cuticular end apparatus similar to that in tylenchid nematodes (Anderson and Byers 1975; Baldwin et al. 1977).
The anterior eight somatic muscle cells of the body wall extend to a level slightly above the prostom-mesostom transition (about 4 µm from the tip). The tip of each muscle is attached to the adjacent hypodermis by denser and more filamentous external laminas and the hypodermis has bundles of tonofilaments traversing its cytoplasm between densities on inner and outer cell membranes. This attachment occurs just at the outer posterior edge of the lips (Figs. 2 and 4).
Observations on moulting
The present report does not consider in detail all of the aspects of formation of the buccal capsule but aims at determining the manner in which various cells participate in this process. Early stages in the moulting process (as identified by Singh and Sulston (1978)) in which secretory activity is detectable in hypodermal seam cells show that the original cuticle separates from the body wall (apolysis), and new cuticle is laid down, closely following the contours of the hypodermal cell membrane. At the same time the hypodermal cytoplasm in the tip of the head retracts so that no cell processes extend beyond the anterior lip-like rim of the prostom (Fig. 7). Retraction of this cytoplasm results in deepening of the groove in the inner surface of cheilostom cuticle just before its contact with prostom cuticle. New body wall cuticle forms along the outer surface of the retracted hypodermal cytoplasm, which appears to be rolled inwards, with a deep groove formed between hypodermal cytoplasm and arcade cytoplasm (Fig. 7). At this stage, cuticle of the buccal capsule has not undergone apolysis and both arcade cytoplasm and cells of the esophagus proper are in close contact with the original cuticle. The nematodes continue to feed. Both arcade cytoplasm and esophageal cells contain dense granules in their cytoplasm. As new body cuticle is deposited, the number of granules increases in arcade and esophageal tissues. Neither anterior nor posterior rings of arcade cytoplasm contain identifiable ribosomes or endoplasmic reticulum. However, granules occur in the posteriorly directed cell processes, and arcade cell bodies are enlarged, contain granular endoplasmic reticulum, and their Golgi apparati have associated dense granules. Similarly, there appear to be more cytoplasmic ribosomes in the epithelial cells of the esophagus. Small clusters of vesicles (perhaps Golgi apparati), but lacking dense granules, do occur in the epithelial cells. However, granules are more prominent in their cell processes that project backward to cell bodies lying within the limit of the esophagus (Albertson and Thomson 1976), These may be the major site of granule synthesis in epithelial cells and arcade tissue. Granular endoplasmic reticulum and cytoplasmic ribosomes become prominent in both muscle cells and marginal cells of the remainder of the esophagus but myofilaments remain throughout the moulting period.
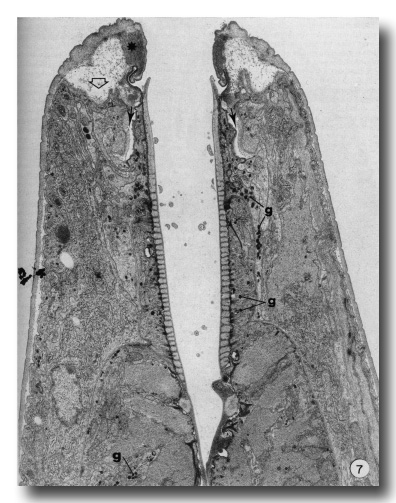 Figure 7. An early stage in the L4 to adult moult. Hypodermis from the head has retracted from its cuticle (open arrow). Note position of dense material (asterisk) in cheilostom cuticle and deepened cheilostom groove. Small arrows indicate the deep groove formed between arcade cytoplasm and hypodermis. Granules (g) are evident in arcade tissue, epithelial cells and muscle cells of the esophagus, x 12000.
Figure 7. An early stage in the L4 to adult moult. Hypodermis from the head has retracted from its cuticle (open arrow). Note position of dense material (asterisk) in cheilostom cuticle and deepened cheilostom groove. Small arrows indicate the deep groove formed between arcade cytoplasm and hypodermis. Granules (g) are evident in arcade tissue, epithelial cells and muscle cells of the esophagus, x 12000.
The membrane of arcade tissues finally separates from the cuticle leaving a clear space. Similarly, the membrane of epithelial cells, marginal cells, and muscle cells of the esophagus separates at small areas as hemidesmosome connections along the membrane disappear.
Later stages of moulting are characterised by a general lethargy in the animal and a lack of esophageal activity as seen in contractions of the posterior bulb. At the same time the mouth opening seems to be "closed". New body cuticle formed around the retracted anterior hypodermal cytoplasm is thicker while the old lip cuticle appears to have been inflated (Fig. 8). The previously deepened fold of the cheilostom cuticle has opened out so that this cuticle now bulges inwards leaving only a very narrow channel into the buccal cavity.
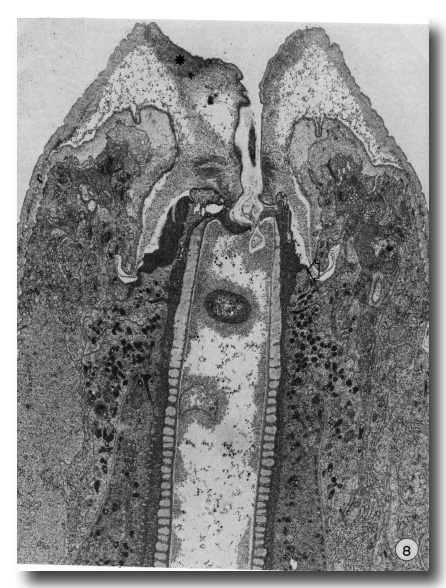 Figure 8. A later stage of moulting when much of the body cuticle has been formed. The mouth is occluded by inflation of old cuticle with loss of the cheilostomal groove and forward movement of densities in cheilostom cuticle (asterisk). Granules are evident in arcade cytoplasm and epithelial cells of esophagus. On the right, anterior arcade tissue has retracted from old prostom cuticle. Arrow notes the anterior limit of arcade tissue which is also the level of the new prostom rim when it is formed later. Dense granular material occurs between old buccal cuticle and cell surfaces x 19 800.
Figure 8. A later stage of moulting when much of the body cuticle has been formed. The mouth is occluded by inflation of old cuticle with loss of the cheilostomal groove and forward movement of densities in cheilostom cuticle (asterisk). Granules are evident in arcade cytoplasm and epithelial cells of esophagus. On the right, anterior arcade tissue has retracted from old prostom cuticle. Arrow notes the anterior limit of arcade tissue which is also the level of the new prostom rim when it is formed later. Dense granular material occurs between old buccal cuticle and cell surfaces x 19 800.
In a later stage, when body cuticle is more completely formed, apolysis of the entire buccal capsule and esophagus cuticle has taken place and the space between the old buccal cuticle and underlying cells is occupied by dense granular material (Fig. 8). Dense granules still occur in both arcade and esophageal cytoplasm. As new buccal and esophageal cuticle forms, the dense granules disappear and the old cuticle becomes progressively thinner. Multivesicular bodies, characteristic of the hypodermis during body wall cuticle formation, do not occur in arcade or esophageal tissue during buccal cuticle formation. After apolysis the contour of the anterior epithelial collar of the esophagus changes so that it tapers more gradually into the esophagus. This appears to be related to the disappearance of tonofilament insertions along the inner epithelial membrane.
Apparently when the mouth closes, the anterior arcade cytoplasm begins to retract from the anterior margin of the prostom cuticle. Thus the newly formed rim of the prostom cuticle occurs posterior to the old, but at a level "appropriate" to the newly forming cheilostom and lip cuticle, being made over the retracted anterior hypodermis (Fig. 8).
Discussion
Although the classical divisions of the rhabditid buccal capsule (i.e. cheilostom, prostom, mesostom, metastom, and telostom) can be recognised at the ultrastructural level in Caenorhabditis elegans, only the distinction between the cheilostom and prostom is marked by a discontinuity of cuticles of different types (i.e. contact of the body wall and esophageal cuticles). Since the cuticle of the rest of the buccal capsule is continuous, showing only internal differences in density, the identification of discrete rhabdions (implying separately formed plates) as composing the walls of the buccal capsule seems untenable. Denser differentiations of the cuticle seem to be related to the degree of attachment of cuticle to underlying cells.
The prostom region is underlain by two rings of syncytial cytoplasm whose cell bodies lie considerably further back, outside the limits of the esophagus as defined by its prominent basal lamina. These can probably be equated with arcade tissue or arcade cells identified earlier in Ascaris (as reviewed in De Coninck 1965). However, similar arcade tissues were not identified in some other nematodes studied recently, notably Nippostrongylus brasiliensis (see Wright 1976). Perhaps the development of this tissue varies in different nematode groups. For example, is it possible that arcade "cells" have given rise to intrinsic musculature of the buccal capsule in the nematodes with "stomatous buccal capsules" (Wright 1976)?
Although contemporary nematologists often refer to the esophagus as being a stomodeum, and hence presumably derived as a rather late embryological process of ectodermal invagination into the developing embryo, it has also been suggested that a true stomodeal invagination may occur only in certain groups (Wright 1976). This may appear in adult forms as an infolding of hypodermis and its attendant body type of cuticle into the functional buccal capsule of the nematode. On this basis, the cheilostom of C. elegans, the only component of the buccal capsule lined by body cuticle, seems to be the only candidate to be considered as a stomodeal invagination. In comparison with other groups (Wright 1976), the cheilostom seems too superficial to be considered a true stomodeum. Recent studies of early embryogenesis in C. elegans (J. E. Sulston, E. Schierenberg, and G. Von Ehrenstein, unpublished data) have found that precursor cells for both esophagus and buccal cavity move inwards from the ventral position with no invagination process. Furthermore, arcade tissues have been found to be lineage related primarily to the esophagus.
Observations on the moulting of the buccal capsule, given here, may also indicate that arcade tissue is more related to esophagus than to hypodermis. The production of body cuticle (apolysis and new cuticle formation) precedes that of the esophagus. Whereas the process of apolysis of body cuticle results simply in the release of the intact body cuticle, apolysis of buccal capsule and esophagus cuticle (prostom as well as the remainder of the buccal cuticle) is followed by extensive breakdown of the cuticle followed by formation of new cuticle. Furthermore, formation of esophageal and buccal cuticle (exclusive of the cheilostom) is characterized by the build up of dense granules in the cuticle forming cells. This characterizes arcade cytoplasm as well as epithelial, muscle, and marginal cells of the esophagus proper, but not body wall hypodermis. Anterior hypodermal cytoplasm retracts from the old cuticle to form the new lip and cheilostom cuticle. Although the anterior arcade cytoplasm also retracts, it does so later, coordinated with apolysis of the esophageal cuticle. It seems that arcade cells do not originate as an ectodermal stomodeum. In the terminology suggested by Wright (1976) C. elegans buccal capsule would be "astomatous."
Although functional aspects of the buccal capsule will be considered separately, it may be noted here that somatic muscles are not modified to operate the buccal capsule as shown in some other nematodes with astomatous buccal capsules (Wright 1976). The attachment of somatic muscles to the body wall at the base of the lips presumably allows these muscles to manipulate the tip of the nematode's head giving delicate, seemingly probing activities resulting in passive bending of the buccal capsule. The flexibility of the buccal cuticle is more apparent in larvae where the length-width ratio of the buccal capsule is greater.
Acknowledgments
Acknowledgement is made of financial assistance of NSERC grant A3757.
References
ALBERTSON, D. G., and J. N. THOMSON. 1976. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. London, Ser. B, 275: 299-325.
ANDERSON, R. V., and J. R. BYERS. 1975. Ultrastructure of the esophageal procorpus in the plant parasite nematode, Tylenchorhynchus dubius, and functional aspects in relation to feeding. Can. J. Zool. 53: 1581-1595.
BALDWIN, J. G., H. HIRSCHMANN, and A. C. TRIANTAPHYL-LOU. 1977. Comparative fine structure of the esophagus of males of Heterodera glycines and Meloidogyne incognita. Nematologica, 23: 239-252.
COOMANS, A. 1963. Stoma structure in members of the dorylaimina. Nematologica, 9: 587-601.
COOMANS, A., L. DE CONINCK, and C. HEIP. 1978. Round table discussions: first workshop on systematics of marine free-living nematodes. Ann. Soc. Zool. Belg. 108:109-113.
DE CONINCK, L. 1965. Structures cephalique and Appareil digestif, cavite buccale. In Traite de zoologie. Edited by P.-P. Grasse. Vol. IV. Masson et Cie, Paris, pp. 127-132,146-162.
DICK, T. A., and K. A. WRIGHT. 1973. The ultrastructure of the cuticle of the nematode Syphacia obvelata (Rudolphi, 1802). II. Modifications of the cuticle in the head end. Can.
J. Zool. 51: 197-202.
RITTER, M. M. 1965. Sous-classe des Secernentea, ordre des Rhabditides. In Traite de zoologie. Edited by P.-P. Grasse. Vol. IV. Masson et Cie, Paris, pp. 732-735.
SINGH, R. N., and J. SULSTON. 1978. Some observations on moulting in Caenorhabditis elegans. Nematologica, 24:63-71.
STEINER, G. 1933. The nematode Cylindrogaster longistoma (Stefanski) Goodey, and its relationship. J. Parasitol. 20: 66-68.
SULSTON, J. E. 1976. Post embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. London, Ser. B, 275: 287-297.
WRIGHT K. A. 1976. Functional organization of the nematode's head. In The organization of nematodes. Edited by N. A. Croll. Academic Press, New York, pp. 71-105.
|