|
The Caenorhabditis elegans Male: Postembryonic Development of Nongonadal Structures
J.E. Sulston D.G. Albertson and J.N. Thomson
M.R.C. Laboratory of Molecular Biology
Hills Road, Cambridge, CB2 2QH, England
Developmental Biology (1980) 78: 542-576
doi: 10.1016/0012-1606(80)90352-8
(Received October 16, 1979; accepted January 18, 1980)
Abstract - Introduction -
Material & Methods -
Results -
Discussion -
Acknowledgments
- References
Abstract
The nongonadal cells in the male nematode Caenorhabditis elegans have been followed through maturation by Nomarski microscopy. Many of the cells are incorporated into the copulatory apparatus, which includes the cloaca, copulatory spicules, sensilla, and musculature. This region has been reconstructed by serial section electron microscopy in order to identify the cell types that arise from known lineages. With the exception of certain bilaterally symmetrical pairs the cells have invariant fates. The development involves a variety of well-defined cell interactions, individual and collective cell movements, cell deaths mediated by designated killers, and the reorganisation of a muscle. The male structures overlie an almost unchanged hermaphrodite tail; their development is more complex than that of the hermaphrodite, and more liable to error.
Introduction
At hatching, the nematode Caenorhabditis elegans contains about 550 nongonadal nuclei; during postembryonic development the number increases to about 810 in the hermaphrodite and to about 970 in the male. The cell lineages leading to these increases were described by Sulston and Horvitz (1977), but at that time the fates of the male specific cells were largely unknown. We have now followed the nongonadal cellular development of the male up to maturity, and have reconstructed the posterior anatomy of the adult from electron micrographs; thus the migration, differentiation, and ultimate fate of each cell in the tail is now known. The development of the gonad has been elucidated by Kimble and Hirsh (1979), and its connection to the somatic structures will be described. We have mapped the nervous system only as far as is necessary to define each cell uniquely.
The development of the male is of interest both for its complexity and for its genetic accessibility. Since the principal sexual form of C. elegans is a self fertilising hermaphrodite, functional males are not required for strain propagation, and male-specific mutants can be maintained without difficulty. Hodgkin (1974) has isolated a number of strains in which hermaphrodites are fertile but males are defective. In another class of mutant, transformer (Klass et al, 1976; Hodgkin and Brenner, 1977), animals bearing two X chromosomes are male rather than hermaphrodite.
The study of such mutant strains is one approach to understanding the control of the developmental events described below. Another approach is selective cell ablation by means of a laser microbeam; experiments using this technique are described both in this and in the following paper.
Materials & Methods
Caenorhabditis elegans, var Bristol, strain N2, was cultured as described by Brenner (1974).
The movements of cells were observed in living animals by Nomarski differential interference contrast microscopy. The animals were mounted on layers of 5% agar, as described by Sulston and Horvitz (1977) except that polyvinylpyrrolidone was not added to the S medium; a green interference filter was always inserted
(1) Standard Mount. The agar was trimmed around the cover slip, and the mount was simply flooded with immersion oil without the application of grease. Unfortunately, this convenient technique cannot be used with the immersion oil currently obtainable (Zeiss 518C), because of its toxicity to the nematodes. For this reason, we have continued to use the old type of oil, containing polychlorinated biphenyls (PCB). If the new type of oil is used, it must be kept out of contact with the agar. A layer of grease does not provide a sufficient barrier. One approach is to employ an 18 X 18 mm cover slip and to keep the oil away from the edges, which are sealed with grease or Voltalef oil (Judith Kimble, personal communication); another, applicable for periods of a few hours, is to use the quick mount, described below.
(2) Quick mount, used when the animal was to be retrieved after a brief examination. A 13-mm-diameter circular cover slip, coated with bacteria in the usual way, was placed over the nematodes; the agar was not trimmed, but covered with a 25-mm square of Saran Wrap having an 11-mm- diameter hole. Only a tiny drop of immersion oil was added, so that the plastic was not wetted by it. For recovery, the Saran Wrap was peeled off, and then the cover slip was raised, the animal being watched to ensure that it remained on the agar; if necessary, the cover slip was lowered and raised until it did so (extra fluid helped).
(3) Anaesthetic mount, used for laser surgery and for drawing with the camera lucida. The nematode was anaesthetised to the required extent in 0.5-1% l-phenoxy-2- propanol and then mounted on agar containing 0.2% l-phenoxy-2-propanol; by this procedure, anaesthesia is rapid (0.5-5 min,depending upon the age of the animal) yet controllable, and the animals do not deteriorate as fast as they do when mounted in more concentrated l-phenoxy-2-propanol. The quick mount procedure was followed, except that no bacteria were applied to the cover slip.
(4) Invertible mount, used to follow certain cells through lethargi (the periods of inactivity during moults), when animals frequently turn over. It was particularly useful in tracking the muscle cells, which are obscured contralaterally by the intestine, through L3 lethargus.
To allow inversion, it is necessary to mount the animal in a very thin layer of aqueous medium; this in turn necessitates a plastic cover film, to permit gas exchange. The cover film was prepared in advance by coating a horizontal 38 x 76 mm microscope slide with 1.5 ml of 2% celloidin (Gurr, London) in amyl acetate. The slide was left for several days in a dustfree place, and the film was then cut into 10 x 10 mm squares; when required, a square was peeled off and the centre was lightly smeared with bacteria. In order to prepare the mount itself, a 22 x 50 mm coverslip was placed between two others raised 60 µm with Ofrex tape, all three being held onto a sheet of plate glass by films of water. A drop of hot 1% agarose was placed on the central coverslip and flattened by means of a siliconised slide supported on the outer coverslips. After cooling, the slide was slid away and the agarose was allowed to dry to about 40 µm (judged by experience; the final thickness of the mount was measured using the calibrated fine focus control). A very small drop of S medium containing the nematode was added, followed by the cover film. The edges of the agarose were allowed to dry completely, and then the mount was flooded with immersion oil. For convenience of handling, it was clamped in a metal frame (35 x 75 x 3 mm). Such mounts lasted for several hours before dehydration began to compress the nematode unduly; by that time the animal had moulted and was remounted in the normal way. PCB immersion oil was used for invertible mounts, but, perhaps on account of the limited exposure period, the new oil can also be employed. Under PCB oil, the upper surface of the celloidin slowly becomes beaded with water droplets; these can be cleared by wiping very lightly with a shred of lens tissue.
Individual animals were always handled by pipette; 1.5 mm tubing was drawn in a flame, and cut to give a tip diameter of 0.2-0.3 mm. The pipette was attached to a mouth tube and kept partly filled with buffer during use. Provided that animals were not sucked into the wide part of the pipette, they never became trapped inside.
Drawings of anaesthetised animals were made with the help of a Zeiss camera lucida. Approximate depths of the nuclei in the animal were recorded by means of a colour code. Stereo pairs of the proctodeal nuclei were made by manual tracing, the spacing of the images of each nucleus being adjusted according to the colour code.
Developmental ages of animals are given in hours from the time of hatching at 20°C, according to the time scale of Sulston and Horvitz (1977).
Animals were prepared for electron microscopy in various ways. For bulk fixation, a male culture plate from which the bacteria had cleared was kept at 4°C overnight and then left for a few minutes in a covered dish containing some dry ice. The plate was inverted over 2% osmium tetroxide for 1-2 min, and the nematodes were washed off into 1% osmium tetroxide. After fixation for 1 hr, straight animals were selected and dehydrated and embedded as described by Ward et al. (1975). Series 4 and 5 (Table 1) were obtained in this way. For series 3 the animal was treated similarly, but was straightened manually after anaesthesia with carbon dioxide. For series 1 and 2 the animals were transferred directly from the Nomarski microscope to 1% osmium tetroxide, to ensure that development was arrested at a known stage.
TABLE 1
ELECTRON MICROGRAPHIC SERIES
| Series |
Age of animal |
Cells lineaged |
| 1 |
42 hr |
Left R3-R9; right R5-R9; dorsorectal ganglion |
| 2 |
43 hr |
- |
| 3 |
Young adult |
Preanal ganglion |
| 4 |
Old adult |
- |
| 5 |
Old adult |
- |
In areas where the morphology and arrangement of cells is well defined (mesoderm, most of the proctodeum, part of the preanal ganglion), the assignment of cell fate did not require the electron microscopic reconstruction of animals of known lineage. This was helpful, because the lineage of only a limited number of cells can be watched in a single individual, and anatomical reconstruction is a time consuming operation. To supply the missing information, two animals were analysed by electron microscopy after particular lineages had been followed (Table 1).
A laser microbeam system developed by John White was used to kill individual cells, as described in the following paper.
The nomenclature used to describe cell lineages is the same as that employed by Sulston and Horvitz (1977). After a cell division, each daughter is given its parent's name followed by a letter representing its relative position: a, anterior; p, posterior; d, dorsal; v, ventral; l, left; r, right. Thus, Rn.aa is the anterior daughter of the anterior daughter of Rn. In the lineage charts, divisions are anterior (to the left) posterior (to the right) unless otherwise designated.
Because many cell lineages are unknown, and some are ambiguous, it is desirable to name cells also in terms of their differentiated characters. The nomenclature for neurons was devised by John White. In his system, every cell is given a name consisting either of three upper case letters or of two upper case letters and a number; the second alternative is used only for groups of similar cells, each cell in a given group having the same letters but a different number. The three-character name is unique except in the case of radially symmetric groups, of which the members appear to be identical: in such a group, every cell has the same three-character name, followed by L (left), R (right), D (dorsal), or V (ventral). Supporting cells in the nervous system, and some other structural cells, are named by a slight modification of the neuron system. In the figures, these names are distinguished from lineage designations by being printed in Gothic type. For all the components of a given sensillum, the same initial pair of letters is used, i.e.-HO, hook sensillum; PC, postcloacal sensillum; PH, phasmid; Rn, ray number n; SP, spicule. The next one or two letters indicate the nature of the component, as follows-sh, sheath cell; so, socket cell; st, structural cell; A, B, C, D or V, neuron. For bilaterally paired sensilla, the name ends in L (left) or R (right). The correspondence between lineal names and systematic names is shown in the lineage charts and drawings.
Results
Outline
Hypodermis
Shaping of the tail
Tail Seam
Proctodeum
Asymmetric cell deaths
Spicules
Gubernaculum
Vas deferens
Nervous system
Ganglia
Sensilla
Lumbar ganglia
Phasmids
Sensory rays
Dorsorectal ganglia
Preanal ganglion
Ventral cord
Mesoderm
Symmetrical assignments
Asymmetrical assignments
Morphogenesis
Juvenile cells
Outline
The general layout of the external features and the copulatory apparatus is shown in figure 1. The nervous system and the mesoderm are shown separately in Figs. 11 and 25. The first section of the results (Hypodermis) will deal with the overall shaping of the tail and the formation of the cuticular fan. The second (Proctodeum) will describe the internal copulatory apparatus: the union between the vas deferens and the alimentary tract, and the formation of the copulatory spicules. The third section covers the nervous system, including the sensilla shown in figure 1, and the fourth the mesoderm.
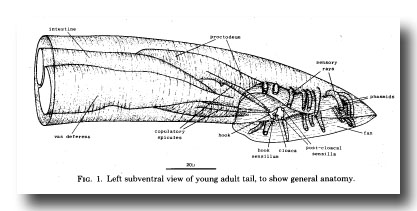 Figure 1. Left subventral view of young adult tail, to show general anatomy.
Figure 1. Left subventral view of young adult tail, to show general anatomy.
Hypodermis
Shaping of the tail. The adult male lacks the tapering tail characteristic of male larvae and hermaphrodites; during maturation, the tail cells withdraw anteriorly and, at the same time, lay down the cuticle of the fan. From the L2 stage onwards the tail of the male is increasingly swollen compared with that of the hermaphrodite, apparently on account of the additional cells which are generated within it. Before and during L4 lethargus, however, there are more rapid changes in shape, which seem to be due to movements of the hypodermis (Figs. 2, 3, 4). In mid-L4, the three hypodermal cells which occupy the posterior part of the tail begin to retreat anteriorly, together with their four nuclei. Some 5 hr before ecdysis, new cuticle can be seen around the retracted cytoplasm. The tips of the sensory rays, already embedded in the outer layer of the adult cuticle, are clearly visible. The forward movement of the tail hypodermis continues, and now the lateral hypodermis, the ray cell bodies, and the dorsal parts of the proctodeum also begin to move anteriorly. Dorsally and ventrally the adult cuticle remains in close contact with the hypodermis, but laterally a wide gap opens between them; this gap is bridged by the rays, which remain attached to the outer layer of cuticle and are spun out behind the retreating cytoplasm. Next, the inner layer of the adult cuticle is laid down, and the lateral extensions of the outer layer collapse around the rays, forming the acellular webbing of the fan.
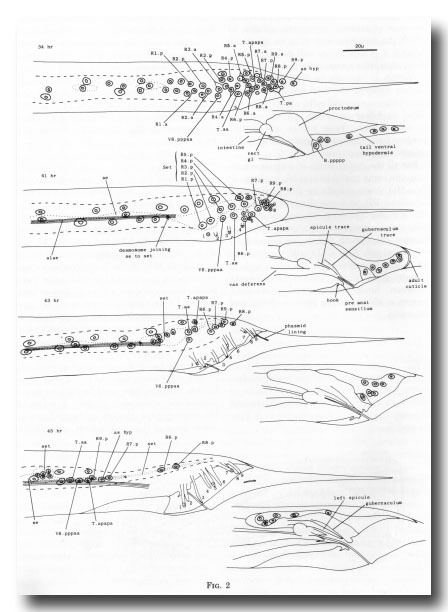 Figure 2. Movement of hypodermal nuclei during tail maturation, left lateral view. The left-hand series of drawings shows the left lateral hypodermis and the right-hand series shows the tail ventral hypodermis. 34 hr: The hypodermal cells (Rn.p) derived from the ray precursor cells are not easily distinguished, other than by their characteristic positions, from the neuroblasts with which they are mingled. Dashed line shows edge of lateral hypodermis at surface of animal; dotted line shows edge of seam cells. 41 hr: The hypodermal cells of the ray groups now lie superficially to the neurons, which are not drawn. The outermost layer of adult cuticle has been laid down, complete with longitudinal alae and tips of the rays (numbered). The first traces of spicule and gubernacular cuticle can be seen. The body seam (se) and tail seam (set) are linked by a desmosome; alae are formed only by the former. At this stage, hypodermal nuclei can still be identified by position. The cells have retreated from the tapering tail. 43 hr: General forward movement, lengthening of the rays. L4 cuticle lining the phasmid channels is pulled out. Tail seam is shrinking and moving forwards. 45 hr: The animal is ready for ecdysis. Rays are fully extended and the fan has collapsed around them. The shrunken tail seam nuclei lie in a bag of cytoplasm at the anterior end of a long process; the body seam still runs back separately to the desmosome. The tail ventral hypodermis has largely withdrawn to lie dorsally to the proctodeum; as hyp, asymmetric hypodermal nucleus; rect gl, rectal gland.
Figure 2. Movement of hypodermal nuclei during tail maturation, left lateral view. The left-hand series of drawings shows the left lateral hypodermis and the right-hand series shows the tail ventral hypodermis. 34 hr: The hypodermal cells (Rn.p) derived from the ray precursor cells are not easily distinguished, other than by their characteristic positions, from the neuroblasts with which they are mingled. Dashed line shows edge of lateral hypodermis at surface of animal; dotted line shows edge of seam cells. 41 hr: The hypodermal cells of the ray groups now lie superficially to the neurons, which are not drawn. The outermost layer of adult cuticle has been laid down, complete with longitudinal alae and tips of the rays (numbered). The first traces of spicule and gubernacular cuticle can be seen. The body seam (se) and tail seam (set) are linked by a desmosome; alae are formed only by the former. At this stage, hypodermal nuclei can still be identified by position. The cells have retreated from the tapering tail. 43 hr: General forward movement, lengthening of the rays. L4 cuticle lining the phasmid channels is pulled out. Tail seam is shrinking and moving forwards. 45 hr: The animal is ready for ecdysis. Rays are fully extended and the fan has collapsed around them. The shrunken tail seam nuclei lie in a bag of cytoplasm at the anterior end of a long process; the body seam still runs back separately to the desmosome. The tail ventral hypodermis has largely withdrawn to lie dorsally to the proctodeum; as hyp, asymmetric hypodermal nucleus; rect gl, rectal gland.
 Figure 3. Maturation of the tail, Nomarski optics. Left-hand views. Bar = 20 µm.
(a) 40 hr. Focus in midline. First signs of adult cuticle, ctl, cuticle; hs, hook sensillum; in, intestine, (b) 41 hr. Focus in left hypodermis. Tips of rays visible; note unique appearance of 6. Golgi activity in body seam (se) and tail seam (set), dotted outline. (c) 43 hr. Focus just below adult cuticle. Rays extending as cells retreat anteriorly. ph, phasmid. (d) 44 hr. Focus in adult cuticle. Rays full length, fan folding.
Figure 3. Maturation of the tail, Nomarski optics. Left-hand views. Bar = 20 µm.
(a) 40 hr. Focus in midline. First signs of adult cuticle, ctl, cuticle; hs, hook sensillum; in, intestine, (b) 41 hr. Focus in left hypodermis. Tips of rays visible; note unique appearance of 6. Golgi activity in body seam (se) and tail seam (set), dotted outline. (c) 43 hr. Focus just below adult cuticle. Rays extending as cells retreat anteriorly. ph, phasmid. (d) 44 hr. Focus in adult cuticle. Rays full length, fan folding.
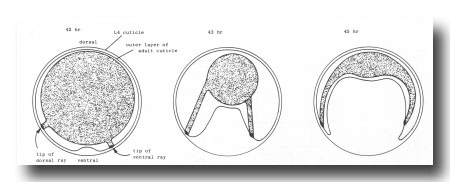 Figure 4. Folding of outer layer of adult cuticle to form the fan. The hypothetical transverse section includes both a dorsal and a ventral ray (viz., rays whose tips lie on the dorsal or ventral surface, respectively, of the mature fan).
Figure 4. Folding of outer layer of adult cuticle to form the fan. The hypothetical transverse section includes both a dorsal and a ventral ray (viz., rays whose tips lie on the dorsal or ventral surface, respectively, of the mature fan).
The preanal region is the last to reorganise; there is a general forward movement of the hypodermis, and spasmodic contractions of the newly formed diagonal muscles pull the ventral side away from the cuticle.
Tail seam. Seam cells are lateral hypodermal cells which do not fuse with the large hypodermal syncytium, divide repeatedly in larvae, generate alae, and possess large Golgi bodies during cuticle secretion. In the L4 the seam cells, having ceased to divide, fuse together into a continuous band on each side; after the L4 moult the seam appears to atrophy (John White, unpublished results; Sulston and Horvitz, 1977; Singh and Sulston, 1978).
In the male the three most posterior seam cells (V5, V6, and T) are diverted into the production of the rays (figure 17). However, in the late L4, the posterior daughters of the ray precursor cells Rl, R2, R3, R4, and R5 fuse together and appear seamlike in their possession of large Golgi bodies (figure 3); we therefore call them the tail seam (set). The tail seam does not produce alae (Figs. 2 and 3), but it does shrink during L4 lethargus and leaves a narrow superficial band which forms a rearward extension of the body seam. The two seams never fuse, but remain attached to one another by a desmosome at their original point of contact (figure 5). Usually, all five nuclei of the tail seam are transported anteriorly in a bag of cytoplasm which remains connected via a narrow process to the posterior part (figure 2). However, in one animal electron microscopical reconstruction revealed that on each side one of the nuclei had remained posteriorly.
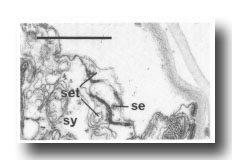 Figure 5. Desmosome joining body seam (se) to tail seam (set), sy, large hypodermal syncytium. Electron micrograph of transverse section, series 4. Bar = 1 µm.
Figure 5. Desmosome joining body seam (se) to tail seam (set), sy, large hypodermal syncytium. Electron micrograph of transverse section, series 4. Bar = 1 µm.
The function of the tail seam was investigated by ablation of its constituent cells soon after their birth in four animals. No other hypodermal cells replaced them, and the body seam did not grow posteriorly. The fan was somewhat attenuated, but electron microscopy revealed that the cuticle had collapsed to form it in the usual way. Thus, no essential role for the tail seam was identified.
It appears that the posterior daughters of R6, R7, R8, and R9 fuse with the large hypodermal syncytium. In series 1, fixed at a time when the nuclei are still identifiable by their positions, only R8.p and R9.p are separate cells. In two adults examined by electron microscopy (series 4 and 5), all the posterior lateral hypodermal nuclei were found to lie either in the seams or in the large syncytium.
Proctodeum
The proctodeum is the junction between the alimentary and genital tracts, together with the copulatory spicules and the spicule channels. The blast cells involved in its formation are B, C, E, F, and K (figure 6). K is an asymmetric blast cell, which lies to the left of the rectum and divides once in the L1 (Sulston and Horvitz, 1977); its anterior daughter resembles the corresponding rectal hypodermal cell on the right, which does not divide. The latter was not named previously, and is now designated K'.
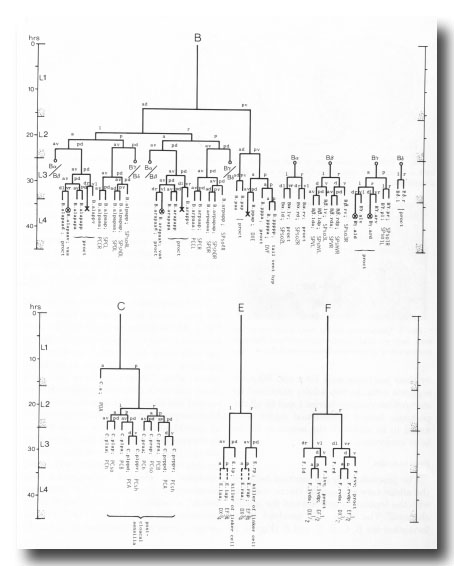 Figure 6. Lineages of cells which divide only in the male. Circled crosses identify pairs of cells in which either the left-hand or the right-hand partner may die. proct, structural cells of proctodeum (figure 8); vas, participates in connection of vas to cloaca.
Figure 6. Lineages of cells which divide only in the male. Circled crosses identify pairs of cells in which either the left-hand or the right-hand partner may die. proct, structural cells of proctodeum (figure 8); vas, participates in connection of vas to cloaca.
The arrangement of nuclei in and around the proctodeum at two stages is shown in figure 7. At the earlier time (34 hr), the divisions are already complete; then, in the L4, the cells move progressively to their final positions. Some remain near their birthplaces, while others migrate to new locations, but their behavior is essentially invariant and bilaterally symmetrical. During L4 lethargus there is little further migration of individual cells, but the entire proctodeum stretches anteriorly (see under Mesoderm) (figure 2). At the same time, the cell boundaries become irregular and easily confused in electron micrographs with membranous fragments in the cytoplasm; indeed, a number of the cells seem to degenerate after the L4 moult. For this reason series 1, from an animal fixed at 42 hr, was used for the reconstruction of the proctodeum. Because of the invariant arrangement of nuclei, all the cells could be identified without difficulty (figure 8).
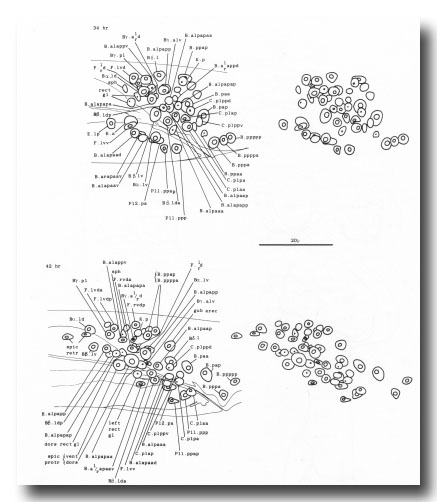 Figure 7. Arrangement of nuclei in proctodeum, left cloacal ganglion, and dorsorectal ganglion in the L4 stage, left lateral view, stereo pairs. Only left and central nuclei are shown. These three regions are combined in a single drawing, because they were treated as a unit when cells were followed by light microscopy. The positions of the nuclei are largely invariant, especially at the later time, when they have stopped moving; at this time some of the muscle nuclei are also shown in their characteristic positions. (N.B. In Sulston and Horvitz, 1977, figure 21, 38 hr: cell pairs B.alpaaa/B.alpaap and Bβ.lda/Bβ.ldp were inadvertently reversed.)
Figure 7. Arrangement of nuclei in proctodeum, left cloacal ganglion, and dorsorectal ganglion in the L4 stage, left lateral view, stereo pairs. Only left and central nuclei are shown. These three regions are combined in a single drawing, because they were treated as a unit when cells were followed by light microscopy. The positions of the nuclei are largely invariant, especially at the later time, when they have stopped moving; at this time some of the muscle nuclei are also shown in their characteristic positions. (N.B. In Sulston and Horvitz, 1977, figure 21, 38 hr: cell pairs B.alpaaa/B.alpaap and Bβ.lda/Bβ.ldp were inadvertently reversed.)
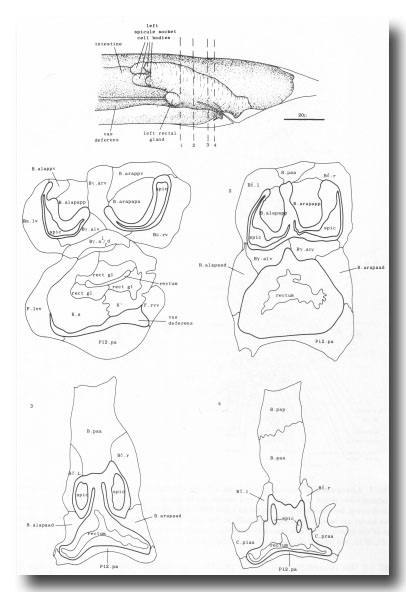 Figure 8. Arrangement of cells in proctodeum at 42 hr (series 1). Cell outlines were traced from electron micrographs of transverse sections, at levels shown in left-hand perspective view at top. Outlines of the cloaca, spicules, and spicule channels are drawn boldly. At this stage the rectum is simply a tube of L4 cuticle. Scale refers to perspective drawing only.
Figure 8. Arrangement of cells in proctodeum at 42 hr (series 1). Cell outlines were traced from electron micrographs of transverse sections, at levels shown in left-hand perspective view at top. Outlines of the cloaca, spicules, and spicule channels are drawn boldly. At this stage the rectum is simply a tube of L4 cuticle. Scale refers to perspective drawing only.
Asymmetric cell deaths. There are two asymmetric deaths in the B lineage (figure 6). They involve the symmetrical pairs of cells Bγ.ald/Bgγ.ard and B.alapaav/B.arapaav (shown X in figure 6): in each pair, one member dies and the other survives. Before the deaths, the members of each pair lie close together in the midline of the animal; it is therefore likely that the two cells are potentially identical but have to compete for a unique fate.
Light microscopic observation, confirmed by electron micrographic reconstruction of one animal, indicates that B.alapaav or B.arapaav dies after being engulfed by P12.pa. After ablation of P12.pa, both B.alapaav and B.arapaav survive.
The dying member of the pair Bγ.ald/ Bγ.ard is often closely associated with F.ld and F.rd; in series l, F.ld and F.rd were found to be fused together and the dying cell lay within the resulting syncytium. However, ablation of F.ld and F.rd has not always prevented the death of Bγ.a'/ver1/rd and their role remains uncertain at present.
Spicules. The cuticle of each spicule is laid down around a bundle of processes derived from neurons and supporting cells (see under Nervous System) (figure 19). The U-shaped profile of the assembly seems to be determined in part by a row of structural cells which protrudes into the channel (figure 8).
Gubernaculum. A strip of thick, sclerotic cuticle is laid down in the roof of the proctodeum by the ventral edge of B.paa (with possible contributions from Bδ.l and Bδ.r). This structure is termed the gubernaculum (Chitwood and Chitwood, 1974) (Figs. 2 and 25c); presumably it serves to guide the tips of the spicules ventrally through the cloaca.
Vas deferens. During the L4 stage the gonad grows posteriorly along the ventral side of the body cavity. In the late L4, the most posterior cell of the gonad, the linker cell (Kimble and Hirsh, 1979), passes between E.lp and E.rp (or their fusion products: see under Preanal ganglion); it is engulfed by one of them and eventually dies (Figs. 9 and 10). Meanwhile, cells K.a and K' grow anteriorly along the outside of the advancing gonad cells and establish contact with them, and B.al/rapaav meets the gonad ventrally. In the absence of E, K.a and K' show no affinity for the gonad, and the connection is not made.
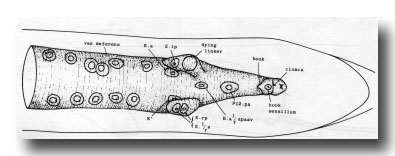 Figure 9. Connection of vas deferens with cloaca, ventral view, mid L4 lethargus.The linker cell has been withdrawn by E.lp in this individual. Unlabelled nuclei are gonadal in origin. B.al/rapaav and p12.pa form the ventral side of the posterior vas; K.a and K' form the lateral and dorsal sides.
Figure 9. Connection of vas deferens with cloaca, ventral view, mid L4 lethargus.The linker cell has been withdrawn by E.lp in this individual. Unlabelled nuclei are gonadal in origin. B.al/rapaav and p12.pa form the ventral side of the posterior vas; K.a and K' form the lateral and dorsal sides.
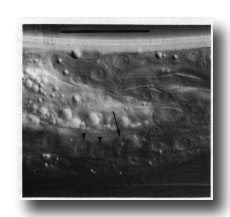 Figure 10. Dying linker cell (arrowed) engulfed by left-hand killer cell whose nuclei E.lp and E.l/r are shown by arrowheads. Left hand view. Bar = 20 µm.
Figure 10. Dying linker cell (arrowed) engulfed by left-hand killer cell whose nuclei E.lp and E.l/r are shown by arrowheads. Left hand view. Bar = 20 µm.
Nervous System
Ganglia. The nervous system of the tail can be divided into a series of ganglia (figure 11). As in the hermaphrodite, there are a pair of lumbar ganglia, a dorsorectal ganglion and a preanal ganglion; all of them contain more cell bodies in the male than they do in the hermaphrodite. In addition, the male has two groups of cell bodies, one on either side of the proctodeum, which we have named the cloacal ganglia. Most of the neuropil is found in the preanal ganglion, to which the other ganglia are connected by a series of commissures.
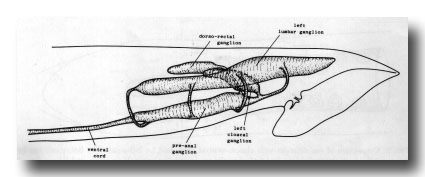 Figure 11. Arrangement of left and central ganglia and principal nerve trunks, left hand view.
Figure 11. Arrangement of left and central ganglia and principal nerve trunks, left hand view.
Sensilla. In describing the sensilla of the male tail, we shall employ the terminology which Ward et al. (1975) used for the sensilla of the head. The latter are present at hatching, and each of them comprises one or more neurons surrounded by two nonneuronal cells, the sheath and the socket. The sheath cell envelops the neurons, to which it is connected by desmosomes. The socket cell encircles the tips of the neurons; it is connected by desmosomes both to the hypodermis and to the sheath cell, but not to the neurons. In contrast, the tail sensilla of the male vary in the number of nonneuronal cells associated with them. They comprise a pair of phasmids (the only tail sensilla in the hermaphrodite), nine pairs of sensory rays, a pair of postcloacal sensilla, a pair of copulatory spicules, and a single sensillum in the sclerotic hook anterior to the cloaca. Their locations are shown in figure 1.
Lumbar ganglia. The lumbar ganglia (Figs. 11 and 12) contain the neurons and supporting cells of the phasmids and the rays. All the cells which are found in the hermaphrodite are also present in the male, and most of them appear to be morphologically similar in the two sexes. However, explicit lineage correlations have not been attempted for this group of cells in the male, and detailed consideration of them will be deferred to another publication.
The only neurons which do differ substantially in the male are the pair T.pppaa; they retain all the synapses which they possess in the hermaphrodite, and can thereby be recognised, but have many additional ones as well. The supporting cells of the phasmid also differ in the male, as described below.
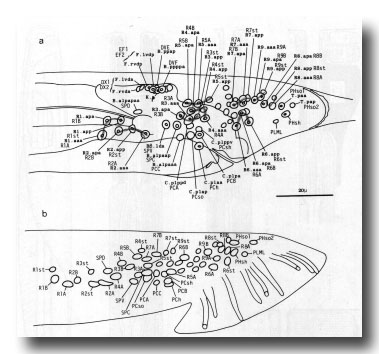 Figure 12. (a) Typical arrangement of nuclei in left lumbar, left cloacal, and dorsorectal ganglion; left lateral view; late L4 (42 hr). Unmarked nuclei belong to juvenile or hermaphrodite groups. Dorsorectal ganglion is compact group of cells at dorsal edge of proctodeum. (b) Typical arrangement of nuclei in left lumbar and left cloacal ganglion; left lateral view; young adult. Positions in cloacal ganglion are essentially invariant at this stage; those in remainder of lumbar ganglion are variable.
Figure 12. (a) Typical arrangement of nuclei in left lumbar, left cloacal, and dorsorectal ganglion; left lateral view; late L4 (42 hr). Unmarked nuclei belong to juvenile or hermaphrodite groups. Dorsorectal ganglion is compact group of cells at dorsal edge of proctodeum. (b) Typical arrangement of nuclei in left lumbar and left cloacal ganglion; left lateral view; young adult. Positions in cloacal ganglion are essentially invariant at this stage; those in remainder of lumbar ganglion are variable.
Phasmids. At the L2 stage, the paired phasmids (Figs. 1 and 13) are identical in the male and the hermaphrodite, each having two neurons, a sheath cell, and two cells which jointly perform the "socket" function. One of these cells, PHso1, wraps around the tips of the neurons in the usual way; it is connected by desmosomes to the sheath and the second cell, PHso2, but not to the hypodermis. PHso2 is connected to PHso1 and the hypodermis, but not to the sheath.
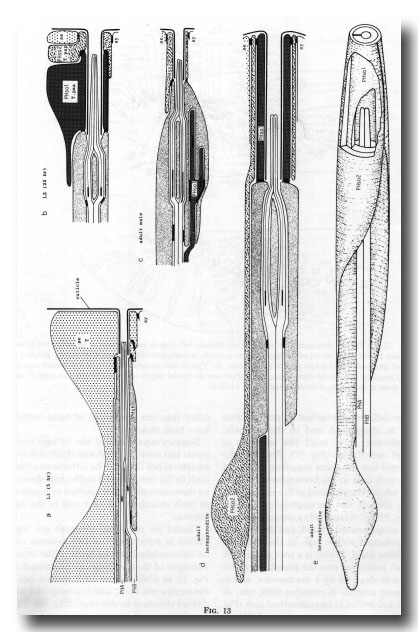 Figure 13. Development of the phasmid; diagrammatic longitudinal sections, (a) 5 hr after hatching. Seam cell T has not completed cytokinesis of the first division. PHA, PHB, phasmid neurons containing basal bodies, (b) Late L2. Hermaphrodite and male phasmids are identical. T.paa wraps around the phasmidial channel; it is attached to itself, the sheath, and T.pap by desmosomes. T.pap is attached in turn to the seam (se) and syncytial (sy) hypodermis, (c) Adult male. The socket is PHso2 (T.pap). (d) Adult hermaphrodite. PHso1 (T.paa) and PHso2 (T.pap) jointly perform the socket function, although PHso2 has become a thin sheet that encircles PHso1. (e) Adult hermaphrodite. The sheath and part of PHso2 have been removed to display PHso1.
Figure 13. Development of the phasmid; diagrammatic longitudinal sections, (a) 5 hr after hatching. Seam cell T has not completed cytokinesis of the first division. PHA, PHB, phasmid neurons containing basal bodies, (b) Late L2. Hermaphrodite and male phasmids are identical. T.paa wraps around the phasmidial channel; it is attached to itself, the sheath, and T.pap by desmosomes. T.pap is attached in turn to the seam (se) and syncytial (sy) hypodermis, (c) Adult male. The socket is PHso2 (T.pap). (d) Adult hermaphrodite. PHso1 (T.paa) and PHso2 (T.pap) jointly perform the socket function, although PHso2 has become a thin sheet that encircles PHso1. (e) Adult hermaphrodite. The sheath and part of PHso2 have been removed to display PHso1.
Reconstruction of a 5-hr-old hermaphrodite revealed no discrete socket cell; instead, the phasmid opened to the outside through seam cell T. It is interesting that the latter meets the definition of a true socket cell, being attached by desmosomes both to the sheath and to the syncytial hypodermis. T is itself the ancestor of PHso1 and PHso2 (figure 17). Probably the phasmid has the same structure in the L1 male as it does in the hermaphrodite.
In adults, the phasmid of the male differs from that of the hermaphrodite. In the latter, PHso2 retains only a tenuous process that wraps around PHso1, and the latter is the principal socket cell. In the male, on the other hand, PHso2 is a true socket cell; PHso1 protrudes into the sheath, to which it is still attached by a desmosome, and in different animals it contains zero, one, or two basal bodies in its ensheathed part (figure 14). No neuronal characteristics of PHso1, other than the possession of basal bodies, have been observed.
 Figure 14. Adult male phasmid, showing basal bodies in neurons (PHA, PHB) and socket cell (PHso1). Electron micrograph of transverse section, series 5. Bar = 1 µm.
Figure 14. Adult male phasmid, showing basal bodies in neurons (PHA, PHB) and socket cell (PHso1). Electron micrograph of transverse section, series 5. Bar = 1 µm.
Sensory rays. Each of the 18 rays comprises two neurons (RnA and RnB) and one structural cell (Rnst). The latter is attached both to the neurons and to the hypodermis by desmosomes; thus it appears to function as both sheath and socket cell to the ray neurons.
Except for ray 6, all the rays are very similar in structure. The two neurons can be distinguished on the basis of the ultrastructure of their endings, as illustrated in figure 15; in addition, the RnB neuron has a distinctive cell body, which is large and has dilated cisternae in the adult (figure 16). The tip of neuron B is surrounded by a small cylinder of cuticle (figure 15, tsl). In ray 6, neuron A resembles the other RnA's; neuron B differs from other RnB's in not having a dark tip, in not obviously reaching the exterior, and in having a compact cell body. By Nomarski optics, the tip of ray 6 never displays the refractile ring and dot characteristic of the other rays (figure 3b and d).
The three cells which generate a given ray are formed from the anterior daughter of a single ray precursor cell Rn (figure 17). The posterior daughter, Rn.p, is a hypodermal cell which fuses either with the large hypodermal syncytium (sy) or with other ray hypodermal cells to form the tail seam (set); the fate of the hypodermal cell from a particular ray precursor cell is invariant.
In the animal chosen for series 1 most of the ray cells were followed until fixation at the beginning of L4 lethargus, when the developing rays could be seen (figure 3); all the rays appeared normal, except for left ray 3, which was missing. Subsequent electron microscopic reconstruction revealed that with this exception Rn.app became the structural cell. This assignment is consistent with the results of laser ablation experiments (Sulston and Horvitz, 1977) which showed that Rn.app is required for ray formation. The absence of left ray 3 was due to the failure of R3.app to form a process, although the neurons were completely differentiated. The loss of a ray is a common occurrence: in a random sample of adults, about half are defective in this way. Usually, the missing ray is 3, 8, or 9. Of the various nonlineaged animals which were reconstructed by electron microscopy, one lacked left ray 3 and another lacked right ray 9; in neither case could the corresponding structural cell be found, although the neurons were present and differentiated in the usual way. Perhaps the structural cells failed to form processes, like left R3.app in series 1, and were swept away by the movement of other cells during the L4 moult.
Cells Rn.aaa and Rn.apa were identifiable as neurons in series 1, but none of them had formed striated rootlets. However, by using the other criteria listed in figure 15, it was possible to distinguish between them in most cases; the results are shown in Table 2. It is very difficult to trace a reasonable number of ray cell bodies through L4 lethargus, on account of the rapid and extensive cell movements which take place. Therefore, we confirmed the assignments for Rn.aaa and Rn.apa by the following experiment.
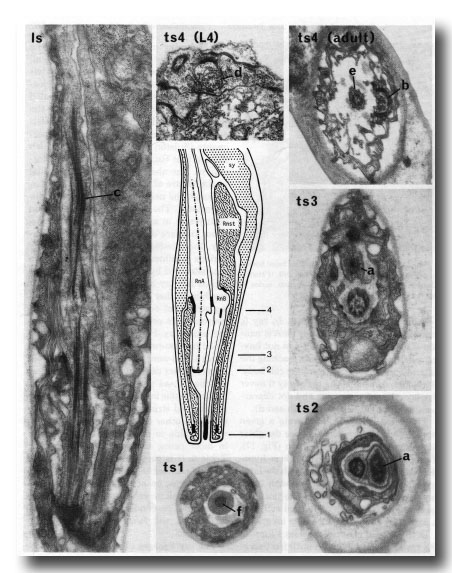 Figure 15. The central drawing represents a longitudinal section through a sensory ray (other than ray 6). The electron micrographs (x 30,000) illustrate the diagnostic criteria (a-f) that were used to distinguish between the neurons (RnA and RnB). Is, longitudinal section; ts 1-4, transverse sections at levels 1-4; Rnst, ray structural cell. RnA has a long striated rootlet (c); it has no distinct basal body, but the broad tip contains darkly staining material which is arranged in a diffuse ring at slightly greater depth (a). RnB projects to the exterior, and has a narrow darkly staining tip (f); it contains a distinct basal body, which is particularly clear in young animals (d). RnA typically lies to one side of the channel, and forms desmosomes to the structural cell more distally than does RnB (b); RnB lies centrally in the channel (e).
Figure 15. The central drawing represents a longitudinal section through a sensory ray (other than ray 6). The electron micrographs (x 30,000) illustrate the diagnostic criteria (a-f) that were used to distinguish between the neurons (RnA and RnB). Is, longitudinal section; ts 1-4, transverse sections at levels 1-4; Rnst, ray structural cell. RnA has a long striated rootlet (c); it has no distinct basal body, but the broad tip contains darkly staining material which is arranged in a diffuse ring at slightly greater depth (a). RnB projects to the exterior, and has a narrow darkly staining tip (f); it contains a distinct basal body, which is particularly clear in young animals (d). RnA typically lies to one side of the channel, and forms desmosomes to the structural cell more distally than does RnB (b); RnB lies centrally in the channel (e).
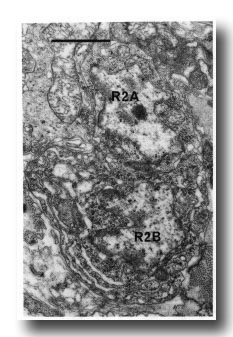 Figure 16. Neuronal cell bodies of sensory ray 2, right side. R2B is larger than R2A, and the cytoplasm contains dilated cisternae. Electron micrograph of transverse section, series 4. Bar = 1 µm.
Figure 16. Neuronal cell bodies of sensory ray 2, right side. R2B is larger than R2A, and the cytoplasm contains dilated cisternae. Electron micrograph of transverse section, series 4. Bar = 1 µm.
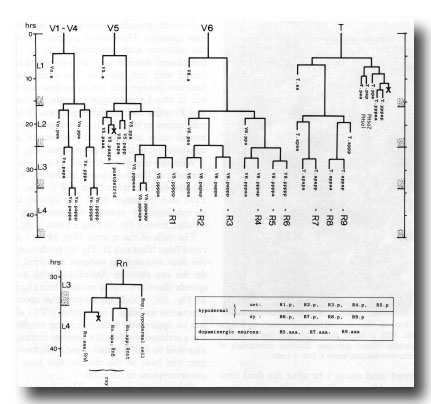 Figure 17. Cell lineages of sensory rays and postdeirid on either side of the animal. In order to clarify the relationships among the ray cells, the ray precursor cells have been redesignated R1-R9.
Figure 17. Cell lineages of sensory rays and postdeirid on either side of the animal. In order to clarify the relationships among the ray cells, the ray precursor cells have been redesignated R1-R9.
Table 2. Correlation between ancestry and morphology of ray neurons in series 1 (42 hr).
The ray lineages of an animal were followed until about 1 hr after the final divisions. All nine Rn.aaa cells were then ablated on the right side (by means of the laser microbeam), and all nine Rn.apa cells were ablated on the left; a minority of other cells were killed accidentally, but care was taken to ensure that none of the desired targets survived. Electron microscopical reconstruction of the resulting adult gave the results shown in Table 3. Five cells on the left formed striated rootlets, which are strong criteria for RnA neurons, while five on the right formed distinct basal bodies, indicative of RnB neurons. With the exception of one cell on the right there were no swollen cell bodies, but this may simply reflect reduced synthetic activity under the conditions of the experiment. We conclude that Rn.aaa cells develop into RnA neurons, and Rn.apa cells into RnB neurons.
Table 3. Correlation between ancestry and morphology of ray neurons in young adult after selective cell ablations.
Cloacal ganglia, postcloacal sensilla, and spicules. The cloacal ganglia contain the neurons and structural cells of the postcloacal sensilla and the neurons associated with the spicules. The correlation between lineage and cell type was carried out in two L4 lethargus animals (series 1 and 2) using the invariant position of the cell bodies as seen in the light microscope at this stage and electron microscopic reconstruction of the cell projections (figure 6).
With the exception of PCC (B.al/rpaaa) the cells of the postcloacal sensillum (figure 18) are derived from blast cell C (figure 6). PCC and its sister SPC (see below) are morphologically similar in possessing a striated rootlet but lacking a basal body.
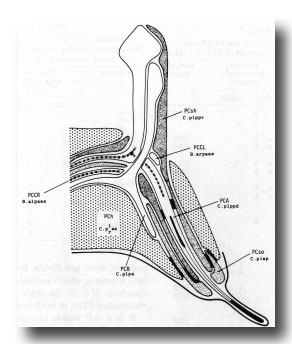 Figure 18. Diagrammatic longitudinal section through the left postcloacal sensillum. The sensory neuron PCA has a short striated rootlet, a basal body, and a dense ending under the cuticle; its dorsal branch projects medially, sometimes crossing to the contralateral nerve cord and running anteriorly. PCB, which also has a dorsal branch, ends in the sheath cell (PCsh). The third neuron, PCC, originates contralaterally and terminates in PCsh; it has a long (7 µm) striated rootlet that ends at the midline, but lacks a basal body. The socket cell, PCso, is attached by desmosomes to PCsh and to the hypodermal cell PCh. The latter is the fusion product of C.plaa and C.praa.
Figure 18. Diagrammatic longitudinal section through the left postcloacal sensillum. The sensory neuron PCA has a short striated rootlet, a basal body, and a dense ending under the cuticle; its dorsal branch projects medially, sometimes crossing to the contralateral nerve cord and running anteriorly. PCB, which also has a dorsal branch, ends in the sheath cell (PCsh). The third neuron, PCC, originates contralaterally and terminates in PCsh; it has a long (7 µm) striated rootlet that ends at the midline, but lacks a basal body. The socket cell, PCso, is attached by desmosomes to PCsh and to the hypodermal cell PCh. The latter is the fusion product of C.plaa and C.praa.
The cells of the spicule (figure 19) are derived from blast cell B (figure 6). In the adult the four socket cells become syncytial, as do the two sheaths. Associated with each spicule there is a motor neuron, (not shown in figure 19), which innervates the spicule protractor muscles. This neuron (SPC) also has an apparently sensory ending, containing a striated rootlet; the sensory ending is attached by half desmosomes to the muscle over the base of the spicule, and may be proprioceptive in function.
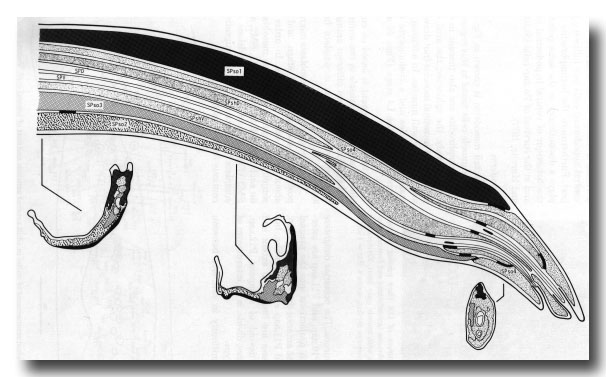 Figure 19. Diagrammatic longitudinal section through spicule of a late L4 male (42 hr, series 1). Tracings of electron micrographs of transverse sections at three levels are included to show the detailed arrangement of the cells. The two ciliated neurons (SPD and SPV) open to the exterior. The two sheaths (SPshD and SPshV) have already begun to fuse at their tips.
Figure 19. Diagrammatic longitudinal section through spicule of a late L4 male (42 hr, series 1). Tracings of electron micrographs of transverse sections at three levels are included to show the detailed arrangement of the cells. The two ciliated neurons (SPD and SPV) open to the exterior. The two sheaths (SPshD and SPshV) have already begun to fuse at their tips.
Dorsorectal ganglion. The dorsorectal ganglion of the male contains, in addition to cells found in the hermaphrodite, neurons from the B and F lineages (Figs. 6, 7, and 12). The positions of the cell bodies are variable and cannot be deduced without following the cell lineages in the light microscope. This was done for one animal (series 1).
The neurons derived from F, whose cell bodies lie in the dorsorectal ganglion, are very similar to those derived from E, whose cell bodies lie in the preanal ganglion (see below). For this reason the two groups will be described together.
F.r/lvda and E.r/laa form a distinctive class, termed DX. They have small, darkly staining cell bodies that send processes along the dorsal edge of the proctodeum and through the basement membrane surrounding it to make electrical junctions with dorsal body muscles (figure 20); the processes sometimes also penetrate into the dorsal cord and run anteriorly. Neurons of no other class have been seen to penetrate basement membranes (White and Albertson, unpublished results).
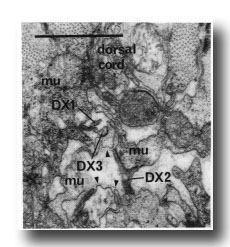 Figure 20. Neurons of Dx class passing through basement membranes(arrowheads) and forming electrical junctions with muscle cells (mu). Electron micrograph of transverse section series 4. Bar = 1 µm.
Figure 20. Neurons of Dx class passing through basement membranes(arrowheads) and forming electrical junctions with muscle cells (mu). Electron micrograph of transverse section series 4. Bar = 1 µm.
F.r/lvdp and E.r/lap form another class, termed EF. These large cells receive synaptic inputs from the rays in the preanal ganglion, and run together anteriorly at the dorsal left corner of the ventral cord; they may continue into the circumpharyngeal nerve ring. However, because they are only partially reconstructed, we cannot be as confident of their assignment to a single class as we are for their sister cells.
The similarity between E and F which is seen in the fates of their progeny extends to their lineages as well (figure 6).
Two neurons from the B lineage, B.ppap and B.ppppa, also have cell bodies in the dorsorectal ganglion. In series 1 a process from B.ppap enters the preanal ganglion on the right, while one from B.ppppa enters on the left. In series 4 two processes from the dorsorectal ganglion, tentatively identified as B.ppap and B.ppppa, both contain large vesicles. Provisionally the cell entering the preanal ganglion on the right has been called DVE (B.ppap) and that entering on the left DVF (B.ppppa).
Preanal ganglion. The preanal ganglion is the group of neurons and supporting cells which lie just anterior to the anus (or cloaca in the mature male); because the ganglion includes motor neurons of the classes found also in the ventral cord, its anterior boundary is somewhat arbitrary (figure 21).
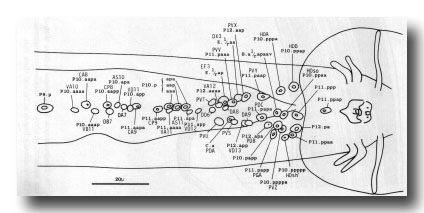 Figure 21. Typical arrangement of the nuclei in adult preanal ganglion and part of ventral cord, ventral view. The preanal ganglion is considered to include P11.aapa and cells posterior to it.
Figure 21. Typical arrangement of the nuclei in adult preanal ganglion and part of ventral cord, ventral view. The preanal ganglion is considered to include P11.aapa and cells posterior to it.
At hatching, the ganglion comprises six neurons. During the L1 stage, divisions of P11 and P12 contribute motor neurons of the ventral cord types (figure 22); the male differs from the hermaphrodite in that P11.aap and P12.aap survive. The male also differs in the division of C (figure 6) whose posterior daughter forms the cells of the postcloacal sensilla (q.v.); in the hermaphrodite C does not divide, but differentiates into a neuron which resembles the anterior daughter of C in the male. The neuron is designated PDA in both sexes.
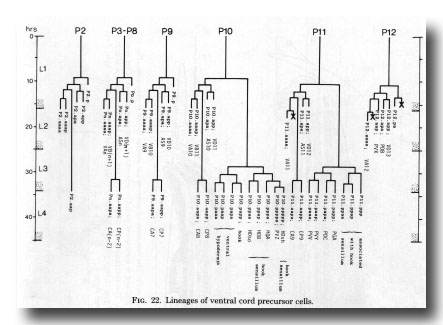 Figure 22. Lineages of ventral cord precursor cells.
Figure 22. Lineages of ventral cord precursor cells.
E is a cell which lies near the anus at hatching and is vestigial in the hermaphrodite. In the male E divides transversely and each of its daughters divides antero-posteriorly. At L3 lethargus either one, or occasionally both, of the anterior daughters migrates forwards into the preanal ganglion and there divides into a pair of neurons (described above under Dorsorectal ganglion). Anterior daughters which fail to move forwards do not divide, and usually fuse with one of the posterior daughters; one of the latter, whether fused or not, kills the gonadal linker cell (see above).
In the L3, P10.p moves posteriorly into the preanal ganglion. Both it and P11.p divide repeatedly and contribute neurons, supporting cells, and ventral hypodermal cells to the ganglion.
The morphology of the cells was studied initially in series 4. Cell assignments were made in series 3 (young adult stage) after the preanal ganglion cells had been traced from hatching. At early L4 lethargus most of the cells are sufficiently well defined in position and morphology to allow comparison between the lineage and fine structure of different individuals; on this basis, the assignments of these fixed cells were found to be identical in series 1. In addition, laser ablation experiments have shown that P10.papp, which is one of the mobile cells, invariably generates the hook (figure 1).
Some of the cells are elements of the hook sensillum (figure 23) and can be characterised by the morphology of their endings in it. However, many of the neurons can be defined uniquely only in terms of their processes and synaptic contacts (figure 24). Cells not shown in these figures are similar in the two sexes, and will be described in detail elsewhere.
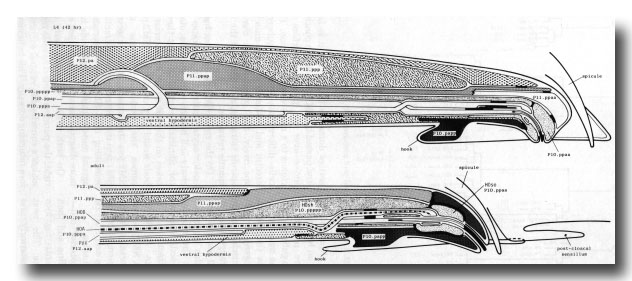 Figure 23. Diagrammatic longitudinal section through the hook sensillum and associated cells. The two sensory neurons are similar to the ray neurons (figure 15), but they are supported by both a socket and a sheath cell. The posterior projection of P12.aap is associated with the neurons: the process runs on their ventral side, and then loops dorsally, passing between P12.pa and P11.ppap (shown in L4 only). The rearrangement of the structural cells in late L4 lethargus is part of the general reorganisation of the preanal ganglion. The cell bodies of Pll.ppap and Pll.ppp change places. The tips of P10.ppaa and P11.ppaa retreat from their initial hypodermal positions, the latter moving so far that it does not appear in the second figure.
Figure 23. Diagrammatic longitudinal section through the hook sensillum and associated cells. The two sensory neurons are similar to the ray neurons (figure 15), but they are supported by both a socket and a sheath cell. The posterior projection of P12.aap is associated with the neurons: the process runs on their ventral side, and then loops dorsally, passing between P12.pa and P11.ppap (shown in L4 only). The rearrangement of the structural cells in late L4 lethargus is part of the general reorganisation of the preanal ganglion. The cell bodies of Pll.ppap and Pll.ppp change places. The tips of P10.ppaa and P11.ppaa retreat from their initial hypodermal positions, the latter moving so far that it does not appear in the second figure.
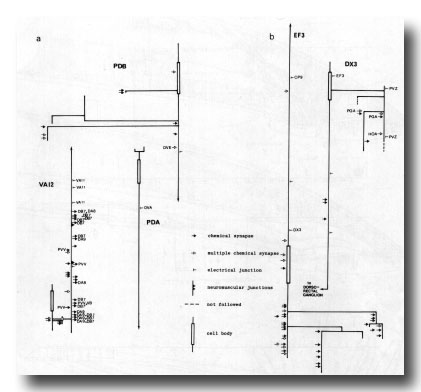 Figure 24. Synaptic maps of neurons in the preanal ganglion. Top is anterior, (a) Cells present in males and hermaphrodites. PDB is modified in the adult male by becoming highly branched in the preanal ganglion. In both males and hermaphrodites its axon runs subventrally on the right to the posterior end of the animal and then runs anteriorly in the dorsal cord. PDA is a dorsal motor neuron and appears similar in the two sexes; however, in the hermaphrodite it is cell C, whilst in the male it is the anterior daughter of C. VA12 is also similar in the two sexes, although it is modified with respect to other VA neurons: few neuromuscular junctions are made, and chemical synapses to DAn and DB7 are frequent, (b) Derivatives of E. EF3 is a large cell that is postsynaptic and branched in the preanal ganglion. It runs anteriorly at the dorsal left edge of the ventral cord. DX3 has a small, darkly staining cell body; its posterior process characteristically runs over the cell body of PVZ and enters the dorsorectal ganglion from the left.
Figure 24. Synaptic maps of neurons in the preanal ganglion. Top is anterior, (a) Cells present in males and hermaphrodites. PDB is modified in the adult male by becoming highly branched in the preanal ganglion. In both males and hermaphrodites its axon runs subventrally on the right to the posterior end of the animal and then runs anteriorly in the dorsal cord. PDA is a dorsal motor neuron and appears similar in the two sexes; however, in the hermaphrodite it is cell C, whilst in the male it is the anterior daughter of C. VA12 is also similar in the two sexes, although it is modified with respect to other VA neurons: few neuromuscular junctions are made, and chemical synapses to DAn and DB7 are frequent, (b) Derivatives of E. EF3 is a large cell that is postsynaptic and branched in the preanal ganglion. It runs anteriorly at the dorsal left edge of the ventral cord. DX3 has a small, darkly staining cell body; its posterior process characteristically runs over the cell body of PVZ and enters the dorsorectal ganglion from the left.
Ventral cord. The ventral cord contains more cell bodies in the male than it does in the hermaphrodite, because of the increased survival of Pn.aap cells and the subsequent division of most of them (figure 22). The male cord has not yet been fully reconstructed, but we have preliminary information about the posterior half. The additional cell bodies can be recognised by their position to the left of the older ones, and by comparison of the ultrastructure of the male cord with that of the hermaphrodite (White et al., 1976). In addition, those Pn.aap derivatives which lie in the preanal ganglion were positively identified in series 3.
The daughters of P7.aap and PS.aap are all motor neurons. The posterior daughters of P9.aap, P10.aap, and P11.aap are interneurons with projections into the preanal ganglion. The anterior daughters of P9.aap, P10.aap, and P11.aap are neuronlike, but their processes are limited in extent and apparently devoid of synapses. P12.aap, which does not divide, is an interneuron of a different type.
The discontinuity in cell fate between P8.aap and P9.aap is interesting, because it parallels a change in the behaviour of the ventral hypodermal cells. Furthermore, the hermaphrodite displays a discontinuity at the same point in the behaviour of ventral hypodermal cells and in the survival of Pn.aap cells (Sulston and Horvitz, 1977).
Mesoderm
The tail mesoderm is shown in figure 25. All the muscle cells found in this region of the hermaphrodite are also present in the male: namely, the sphincter, the anal depressor, the intestinal muscles (not drawn), and the body wall muscles. In addition, 41 male sex muscles and a coelomocyte are formed during the L4 stage (Figs. 26 and 27).
The following account is based on complete lineages of left muscle in two animals and right muscle in two different animals, together with numerous incomplete lineages.
The three pairs of sex mesoblasts migrate posteriorly during the L3 stage, and at about 34 hr begin to divide. In order to simplify description, the three mesoblasts on each side will be called SM1, SM2, and SM3 (Figs. 26 and 27). The divisions are complete by L3 ecdysis, but the migrations continue for several hours.
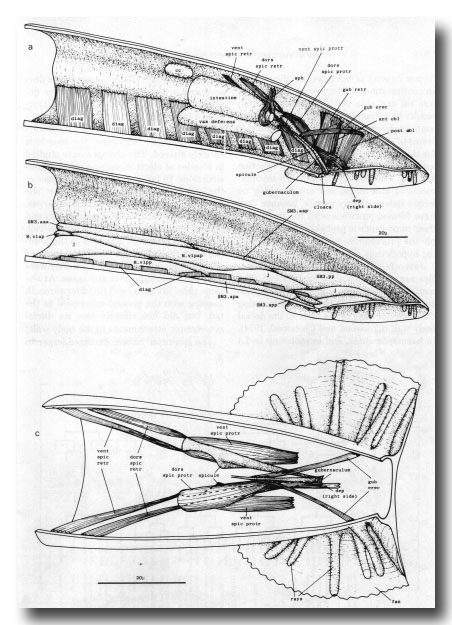 Figure 25. Adult mesoderm. The drawings represent the contractile elements, rather than the cell outlines, of the internal muscles, (a) and (b) Left-hand view of right half of tail, (a) Internal muscles and coelomocyte; the intestinal muscle is omitted, (b) Ventral body wall muscles and ventral attachments of diagonals. SM3 derivatives intercalate with pre-existing body wall muscles; the remaining muscles are present in the hermaphrodite also, and their usual ancestry is indicated, j, juvenile (i.e., present at hatching), (c) Dorsal view of spicule muscles. Right-hand dorsal spicule protractor and arm of depressor (reorganised) have been removed to show spicule and ventral spicule protractor: posterior ends of ventral spicule protractors attach to ventral body wall, ant, anterior; post, posterior; dors, dorsal; vent, ventral; cc, coelomocyte; dep, anal depressor; diag, diagonal; erec, erector; gub, gubernacular; obi, oblique; protr, protractor; retr, retractor; sph, sphincter muscle; spic, spicule.
Figure 25. Adult mesoderm. The drawings represent the contractile elements, rather than the cell outlines, of the internal muscles, (a) and (b) Left-hand view of right half of tail, (a) Internal muscles and coelomocyte; the intestinal muscle is omitted, (b) Ventral body wall muscles and ventral attachments of diagonals. SM3 derivatives intercalate with pre-existing body wall muscles; the remaining muscles are present in the hermaphrodite also, and their usual ancestry is indicated, j, juvenile (i.e., present at hatching), (c) Dorsal view of spicule muscles. Right-hand dorsal spicule protractor and arm of depressor (reorganised) have been removed to show spicule and ventral spicule protractor: posterior ends of ventral spicule protractors attach to ventral body wall, ant, anterior; post, posterior; dors, dorsal; vent, ventral; cc, coelomocyte; dep, anal depressor; diag, diagonal; erec, erector; gub, gubernacular; obi, oblique; protr, protractor; retr, retractor; sph, sphincter muscle; spic, spicule.
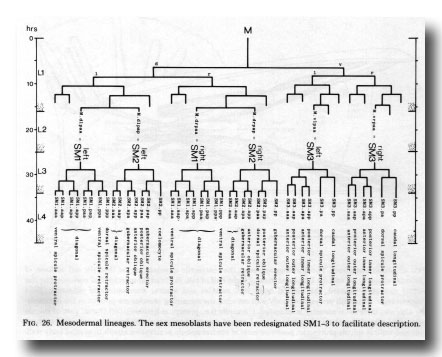 Figure 26. Mesodermal lineages. The sex mesoblasts have been redesignated Sm1-3 to facilitate description.
Figure 26. Mesodermal lineages. The sex mesoblasts have been redesignated Sm1-3 to facilitate description.
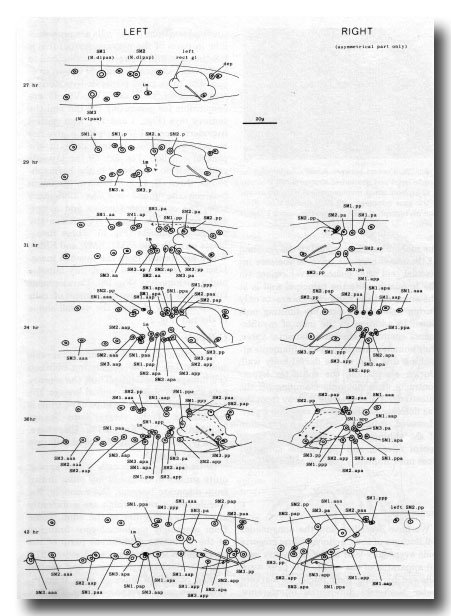 Figure 27. Development of the mesoderm. The entire left-hand side is shown, but only the asymmetric region of the right. Note that the arrangement given previously (Sulston and Horvitz, 1977, figure 26) for the muscle nuclei at 35 hr has proved to be abnormal.
Figure 27. Development of the mesoderm. The entire left-hand side is shown, but only the asymmetric region of the right. Note that the arrangement given previously (Sulston and Horvitz, 1977, figure 26) for the muscle nuclei at 35 hr has proved to be abnormal.
Symmetrical assignments. Most of the cells behave in the same way on both sides. SM2.apa and SM2.app move posteriorly past the cloaca, becoming the gubernacular retractor and anterior oblique muscles, respectively; SM1.aaa becomes the ventral spicule protractor. Some progeny of SM1 and SM2 become diagonal muscles, and do not have a rigidly defined arrangement.
Five of the progeny of SM3 become ventral longitudinal muscles, which move into particular positions next to the existing body muscle (figure 25). The sixth cell becomes the dorsal spicule protractor.
Asymmetrical assignments. At 31 hr (figure 27) three cells - SM2.pp, SM2.pa, and SM1.pp - lie close together dorsally. As the final round of divisions proceeds, SM2.pp and the daughters of SM1.pp and SM2.pa behave differently on the two sides.
On the left, the most posterior cell - SM2.pp - moves to the midline and then anteriorly, ultimately differentiating into the lone dorsal coelomocyte. The next cell - SM2.pap - migrates posteriorly and settles dorsal to the cloaca; it is followed and passed by SM2.paa, which continues to move over the posterior surface of the cloaca to a more ventral position. The two cells differentiate into the gubernacular erector and posterior oblique muscles, respectively. SM1.ppp becomes the dorsal spicule retractor, and SM1.ppa the ventral spicule retractor.
On the right, SM2.pp does not become a coelomocyte; instead, it migrates posteriorly and becomes the gubernacular erector. Similarly, each of the next three cells takes on the role which its anterior neighbour would play on the left: SM2.pap becomes the posterior oblique muscle, SM2.paa the dorsal spicule retractor, and SMl.ppp the ventral spicule retractor. The remaining cell - SM1.paa - becomes a diagonal muscle; thus the right side has one more diagonal muscle than the left side.
On both sides, but particularly on the right, the putative ventral spicule retractor makes a considerable ventral excursion before returning dorsally. This behaviour suggests that it has some tendency to become a diagonal muscle, and indeed the loss of a spicule retractor in this way is a common occurrence after cell ablations (see Sulston et al., 1983).
Morphogenesis. In addition to their role as muscles in the adult, some of these cells play a part in morphogenesis of the proctodeum. If the mesoblast (M) is destroyed in a young L1, the proctodeum develops normally until L4 lethargus but then fails to elongate anterodorsally. The result is a compressed structure containing crumpled spicules and gubernaculum. In series 1 the proctodeal structural cells B.alappv and B.arappv form specialised contacts with the dorsal spicule retractors, a finger from the structural cell being inserted into the muscle (figure 28); there are also darkenings of the cell membranes where B.al/rappv contacts the dorsal spicule protractor. The anterior movement of the retractors, and the fact that B.al/rappv is apparently dragged forward during L4 lethargus (figure 3a), squeezing between B.al/rapapa and Bγ.pl/r, suggests that the former contact is the more significant one. In confirmation of this, loss of both retractors on one side (by cell ablation) often results in a crumpled spicule; however, in some cases one of the spicule protractors seems to grow anteriorly and so compensate for the loss. If a single retractor is lost, the survivor can fork posteriorly and function as both. The retractors have few myofilaments at this stage, and are probably no longer independently mobile, because they have formed junctions with the body wall; their importance is probably that they couple the proctodeum to the anteriorly moving hypodermis.
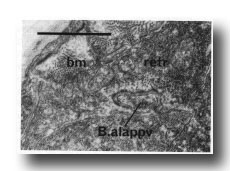 Figure 28. Contact between dorsal spicule retractor muscle (retr) and proctodeal structural cell B.alappv at 42 hr. Note sparse myofilaments in retractor muscle at this time compared with body muscle (bm). Electron micrograph of transverse section, series 1. Bar = 1 µm.
Figure 28. Contact between dorsal spicule retractor muscle (retr) and proctodeal structural cell B.alappv at 42 hr. Note sparse myofilaments in retractor muscle at this time compared with body muscle (bm). Electron micrograph of transverse section, series 1. Bar = 1 µm.
Juvenile cells. Some of the juvenile muscles are modified during maturation. The most striking change is in the anal depressor, which is an H-shaped cell connecting the posterior edge of the anus to the dorsal body wall (Chitwood and Chitwood, 1974). In hermaphrodites, and in males up to L4 lethargus, the myofilaments run in the dorsoventral arms, and contraction of the depressor opens the anus in defaecation. During L4 lethargus in the male, the myofilaments are reoriented to run anteroposteriorly, so that the function of the muscle is entirely altered. The changes were studied in a series of electron microscopical reconstructions. In late L4, the anterior edges of the cell grew forward until they met the dorsal spicule protractors. In mid lethargus, the filaments became detached dorsally and shrank into the ventral half of the cell. They were next seen attached to the anterior edge, thus in a sense forming extensions of the dorsal spicule protractors; they were not seen to disappear at any stage. At the same time, the entire cell tilted forwards, in keeping with the general movement in the tail, but did not entirely lose its dorsal cytoplasmic attachments to the body wall.
The sphincter muscle becomes larger in the male. This corresponds to the function of the intestinorectal valve as the sole intestinal seal after cloacal development (in the hermaphrodite the principal seal is at the anus, and the sphincter contracts only during defecation, possibly limiting outflow). In addition to the loop of myofilaments encircling the intestinorectal valve, the sphincter muscle develops filaments attaching this loop to the dorsal body wall. During ejaculation, the sphincter hyper-contracts, thus not only clamping off the intestine but also drawing it away from the vas deferens so that sperm can pass freely. However, drug-induced hypercontraction of the sphincter does not in itself cause sperm release, and loss of the sphincter does not completely prevent sperm release.
Discussion
Summary
Cell Fate
Sensilla
Comparison with Hermaphrodite Development
Changes in Cell Function During Development
Symmetry
Cell Death
Developmental Variability
Moulding of the Cuticle
Summary
We have described how the nongonadal cell lineages of the Caenorhabditis elegans male eventually lead to the secondary sexual apparatus of the adult. The cells were followed by using their nuclei as markers under Nomarski differential interference contrast optics, and late stages were reconstructed from electron micrographs of serial sections. In muscle and structural elements, the final positions of the cells are reproducible; in parts of the nervous system this is not the case, and the reconstruction had to be carried out on an animal of known lineage. The three most posterior hypodermal seam cells on each side (V5, V6, T) are responsible for generating the fan and the sensory rays (Figs. 1 and 17). Two ventral hypodermal cells, P10.p and P11.p, give rise to neurons and supporting cells in the preanal ganglion (Fig. 22). Of the juvenile cells which do not divide in the hermaphrodite, three (B, E, and F) form the union between the gonad and the alimentary tract, the copulatory spicules and associated neurons; the fourth (C) forms the postcloacal sensilla (Fig. 6). Three mesoblasts on each side (SM1, SM2, and SM3), which are formed from the juvenile mesoblast (M), produce the copulatory muscles and a coelomocyte (Fig. 26). Development culminates in a mass movement of cells which abruptly changes the overall shape of the tail (Fig. 2).
Cell Fate
Inspection of the cell assignments reveals some pattern in the correlation between cell lineage and cell fate. Thus, the sensory rays share a common terminal lineage; the repetitive formation of groups of neurons is reminiscent of the ventral cord lineage (Sulston and Horvitz, 1977). Elsewhere, there is some clustering of related cells into particular sensilla
On the other hand, cells which appear quite similar in the adult are often drawn from disparate lineages. For example, the spicule socket cells, which are sufficiently alike to fuse together in the adult, come from seemingly nonhomologous branches of the B lineage in different parts of the proctodeum (cf. Sulston and Horvitz, 1977, Fig. 21). Similarly the dorsal and ventral spicule protractor muscles arise from diverse lineages at diverse locations. The fact that analogous cells can be well separated spatially at first, and only subsequently assemble at the same location, suggests that their commitment is not an accident of position but rather is programmed into them at birth.
Sensilla
Inspection of Figs. 6 and 22 shows that lineages of sensilla can take a wider variety of forms than those proposed by Sulston and Horvitz (1977). Different tail sensilla contain from one to three supporting cells (in contrast to head sensilla, which invariably contain two). Furthermore, neurons and supporting cells do not necessarily occupy discrete branches of the lineage.
Nevertheless, it is worth searching for common features, because data presented previously (Sulston and Horvitz, 1977) and in the following paper indicate that the terminal branches of a number of lineages are determinate. One generalization that can be made is that sheath cells are more often sisters of neurons than are socket cells. Socket cells are sometimes formed later in development than the other components of sensilla, their function being at first performed by the seam cells from which they eventually arise; this is true of the phasmid and probably also of the deirid (John White, personal communication).
Comparison of the hook and ray sensilla reveals more specific similarities. The pair of neurons which innervate the hook sensillum (HOA and HOB) markedly resemble the pair (RnA and RnB, respectively) which innervate each ray (other than ray 6). It is therefore interesting that the lineage of the hook sensillum can be seen as a permutation of the ray lineage. On this view the programmed cell death in the ray lineage removes the potential ancestor of the sheath and motor neuron formed by the hook sensillum lineage; the ray structural cell would then be the homologue of the hook socket cell.
The cephalic sensilla may perhaps fit into the same pattern. Ward et al. (1975) showed that, in the male, each cephalic sensillum contains a pair of sensory endings: one lies beneath the cuticle, like the solitary, dopaminergic neuron found in the hermaphrodite, whilst the other penetrates to the exterior. The male has four swollen cell bodies, which are absent from the hermaphrodite, near the nerve ring (Sulston and Horvitz, 1977); these "cephalic companions" are thought to be the cell bodies of the four male-specific cephalic processes. Their morphology in the electron microscope is similar to that of RnB and HOB. There is thus an analogy between the cephalic neuron and RnA on the one hand, and between the cephalic companion and RnB on the other. However, the cephalic neuron does lack the striated rootlet typical of RnA. At present the lineages of the cephalic sensilla, which are embryonic, are unknown.
Comparison with Hermaphrodite Development
With few exceptions, all the nongonadal cells of the hermaphrodite are found also in the male, and most of them have similar functions in two sexes.
The only significant absence is that of the vulva and its associated musculature. The blast cells which generate the vulva in the hermaphrodite (P5.p, P6.p, P7.p) do not divide in the male, in which they form part of the ventral hypodermis. The hermaphrodite sex myoblasts (M.vlpaa, Mvrpaa), on the other hand, are also male sex myoblasts (renamed SM3), but their division pattern and the functions of their progeny are different in the two sexes.
The remaining male blast cells divide less, or not at all, in the hermaphrodite, but nevertheless become differentiated cells with well defined functions. In the male, one or more of the progeny of a blast cell may take on the function which their parent performs in the hermaphrodite. Thus, C becomes a neuron in the hermaphrodite and its anterior daughter becomes a similar neuron in the male; the mesoblast Mdlpap (SM2) becomes a coelomocyte in the hermaphrodite whilst M.dlpappp (SM2.pp) does so in the male; B, E, F, and the various lateral and ventral blast cells are all hypodermal cells in the hermaphrodite and except for E each produces hypodermal progeny in the male as well.
For the most part, then, the secondary sexual apparatus of the male overlies the existing hermaphrodite anatomy. This contrasts with the development of the gonad (Kimble and Hirsh, 1979), in which the division patterns of the hermaphrodite and the male diverge at an early stage.
Changes in Cell Function during Development
A number of cells play more than one role in the course of the development of the male, as the following examples show:
(1) The anal depressor muscle acts to open the anus during defaecation of larvae. During the final moult its myofilaments turn through 90°, and the muscle then acts as an accessory to the spicule protractors.
(2) The spicule retractor muscles are required during L4 lethargus for morphogenesis of the proctodeum. This function may not be related to their subsequent muscular nature; probably they simply act as couplers at this point.
(3) The seam cells, which lay down the lateral cuticle at each moult, are also blast cells in both the hermaphrodite and the male. In addition, the most posterior seam cell (T) is the socket cell of the juvenile phasmid, and segregates this function to two of its daughters during its subsequent division.
(4) Cells B, C, E, F, and K are responsible for forming parts of the rectum in larvae. At the same time they divide repeatedly to yield both structural cells and neurons.
Another striking example of changing cell roles is the reversal in information flow along certain neurons of the ventral cord at the L2 stage, described by White et al. (1978).
Symmetry
In the Postembryonic lineages of the hermaphrodite, the two daughters of a transverse division across the midline have equivalent fates (Sulston and Horvitz, 1977). The lineages of an individual male contain several exceptions to this rule, but when several animals are examined it is found that in all but one case the two cells have equal potential.
Thus, in both cases of asymmetric cell death (B.al/rapaav and Bγ.al/rd) either the left or the right cell can die (Fig. 6). The decision may be due to a position effect, the left and right cells being ordered at random as in the case of the Bα/Bβ and Bγ/Bδ pairs (Sulston and Horvitz, 1977). Similarly, we now know that the left and right daughters of E both have the potential to give rise to neuroblasts. This is not to say that all possible outcomes are equally probable: indeed it would be surprising if that were the case, since the animal is inherently asymmetrical at the cellular level. At present, though, not enough individuals have been watched for the probabilities to be estimated.
In a similar way, each individual is asymmetric while the population as a whole is symmetric with regard to the gonadal lineages (Kimble and Hirsh, 1979). The possible exception to the rule, the development of the mesoderm, is intriguing and unexpected. In fact, because this development is difficult to follow, it seemed likely at first that the observations were in error. However, the cell assignments have proved to be perfectly reproducible on each side.
Cell Death
The gonadal linker cell is engulfed by E.lp or E.rp, and dies soon afterwards. That these events are causally related is shown by the ablation of blast cell E, after which operation the linker cell does not die. It is confirmed by the observation that the linker cell survives in gonads which fail to reach the proctodeum, due to genetic defects or to ablation of other gonadal cells (J. Kimble, personal communication).Thus E.lp and E.rp seem to be killer cells directed against the linker. In the same way P12.pa appears to be responsible for engulfing and killing either B.alapaav or B.arapaav, and the fusion product of F.ld and F.rd may be involved in the death of Bγ.ald or Bγ.ard. It is possible that all programmed cell deaths in the nematode involve killer cells. Alison Robertson (personal communication) has found that ventral cord cells which are programmed to die are surrounded by the syncytial hypodermis before cytokinesis is complete; since overt signs of cell death follow only after some 30 min or more, it is likely (though not proved) that the hypodermis is killing them.
Developmental Variability The development of the male is more prone to variation than that of the hermaphrodite.
(1) A common error is the loss of one or more rays, the most vulnerable being 3, 8, and 9; this appears to be due to the failure of the structural cell to form a process. Frequently, in place of ray 3, a thin process can be seen by Nomarski microscopy, suggesting that at least one of the neurons has been laid down in the normal way.
(2) A less common error is the fusion of rays 8 and 9.
(3) The lineage of cell E is variable: either one, or both, or neither of its anterior daughters may move forward into the preanal ganglion and there divide into two neurons.
None of these variations, or errors, is known to influence mating behavior significantly, but they do show that the male copulatory apparatus is constructed in a less precise way than other parts of the nematode.
Moulding of the Cuticle
During the final moult, the adult cuticle is shaped into a membranous fan encircling the ventral surface of the tail. The animal achieves this by secreting a layer of cuticle at a time when the tail is expanded: subsequently, the tail shrinks, and the oversized cuticle collapses in a welldefined way to form the fan (Fig. 4). This stratagem of collapsing a single layered surface into a double layer is analogous to the formation of an insect wing (Seligman et al., 1975), except that the layer is acellular rather than cellular. The collapse might be caused either by withdrawal of fluid from the subcuticular space or by an active contraction of the middle layer of cuticle. Contraction of cuticle secreted by the body seam has been implicated in the maturation of dauer larvae (Singh and Sulston, 1978). With this in mind, the tail seam was ablated in four animals; however, although the fan cuticle was somewhat attenuated by the loss of hypodermis, it still folded correctly.
Another apparent example of the use of cells as temporary moulds for the cuticle is found in the formation of the spicules. In the late L4, the bundle of processes from the socket, sheath, and neuronal cells of each spicule grow along a U-shaped channel (Fig. 8) and sclerotic cuticle is laid down around the bundle. Subsequently, the cells protruding into the channel shrink, leaving the resulting U-shaped spicule free to move.
Acknowledgments
In the course of this work, we have benefited particularly from discussions with Martin Chalfie, Jonathan Hodgkin, Bob Horvitz, Judith Kimble, and John White. We are also grateful to Marilyn Anness for help with electron microscopical reconstruction, and to Judith Daniels and Annette Lenton for help with the figures. D.G.A. was a research fellow of the Muscular Dystrophy Association of America, Inc.
References
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
Chitwood, B. G., and Chitwood, M. B. (1974). "Introduction to Nematology." University Park Press, Baltimore.
Hodgkin, J. A. (1974). "Genetic and Anatomical Aspects of the Caenorhabditis elegans Male," Ph.D. thesis. University of Cambridge, Cambridge, England.
Hodgkin, J. A., and Brenner, S. (1977). Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics 86, 275-287.
Kimble, J., and Hirsh, D. (1979). The Postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Develop. Biol. 70, 396-417.
Klass, M., Wolf, N., and Hirsh, D. (1976). Development of the male reproductive system and sexual transformation in the nematode Caenorhabditis elegans. Develop. Biol. 52, 1-18.
Seligman, I. M., Filshie, B. K., Doy, F. A., and Crossley, A. C. (1975). Hormonal control of morphogenetic cell death of the wing hypodermis in Lucilia cuprina. Tissue Cell 7(2), 281-296.
Singh, R. N., and Sulston, J. E. (1978). Some observations on moulting in Caenorhabditis elegans. Nematologica 24, 63-71.
Sulston, J. E., and Horvitz, H. R. (1977). Postembryonic cell lineages of the nematode Caenorhabditis elegans. Develop. Biol. 56, 110-156.
Ward, S., Thomson, N., White, J. G., and Brenner, S. (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160, 313-338.
White, J. G., Southgate, E., Thomson, J. N., and Brenner, S. (1976). The structure of the ventral nerve cord of Caenorhabditis elegans. Phil. Trans. Roy. Soc. London Ser. B 275, 327-348.
White, J. G., Albertson, D. G., and Anness, M. A. R. (1978). Connectivity changes in a class of moto-neurone during the development of a nematode. Nature (London) 271, 764-766.
|