|
Post-embryonic Cell Lineages of the Nematode,
Caenorhabditis elegans
J.E. Sulston and H.R. Horvitz
M.R.C. Laboratory of Molecular Biology
Hills Road, Cambridge, CB2 2QH, England
Developmental Biology (1977) 56: 110-156
doi: 10.1016/0012-1606(77)90158-0
(Received August 23, 1976; accepted November 4, 1976)
Abstract - Introduction -
Material & Methods -
Results -
Discussion -
Acknowledgments
- References
WA editors' notes:
The cells labeled E and C in this publication were more recently relabeled U and Y respectively.
Updated lineage trees with complete listings of cell names can be viewed via the "Lineage tree" link in WA home page.
The cells labeled "P" in this publication (i.e., postembryonic P blast cells) should not be confused with embryonic P1-P4 cells and their lineages.
Abstract
The number of nongonadal nuclei in the free-living soil nematode Caenorhabditis elegans increases from about 550 in the newly hatched larva to about 810 in the mature hermaphrodite and to about 970 in the mature male. The pattern of cell divisions which leads to this increase is essentially invariant among individuals; rigidly determined cell lineages generate a fixed number of progeny cells of strictly specified fates. These lineages range in length from one to eight sequential divisions and lead to significant developmental changes in the neuronal, muscular, hypodermal, and digestive systems. Frequently, several blast cells follow the same asymmetric program of divisions; lineally equivalent progeny of such cells generally differentiate into functionally equivalent cells. We have determined these cell lineages by direct observation of the divisions, migrations, and deaths of individual cells in living nematodes. Many of the cell lineages are involved in sexual maturation. At hatching, the hermaphrodite and male are almost identical morphologically; by the adult stage, gross anatomical differences are obvious. Some of these sexual differences arise from blast cells whose division patterns are initially identical in the male and in the hermaphrodite but later diverge. In the hermaphrodite, these cells produce structures used in egg-laying and mating, whereas, in the male, they produce morphologically different structures which function before and during copulation. In addition, development of the male involves a number of lineages derived from cells which do not divide in the hermaphrodite. Similar postembryonic developmental events occur in other nematode species.
Introduction
The development of a multicellular organism from a unicellular egg involves a complex pattern of repeated cell divisions. Classical observations of nematode embryogenesis (reviewed by Chitwood and Chitwood, 1974) revealed that in these organisms early development follows a rigidly fixed program, i.e., an invariant pattern of cell divisions produces specific progeny cells which, in turn, give rise to particular parts of the organism. These and similar studies led to the concept of "cell lineages," in which the ancestry of different organs can be traced back to specific progenitor cells and, ultimately, to the egg (e.g., Wilson, 1925).
In this paper, we extend these observations from the embryonic to the postembryonic period. Many cell divisions occur in the nematode Caenorhabditis elegans after hatching. As in embryogenesis, the pattern of these divisions is rigidly determined; essentially invariant postembryonic cell lineages generate fixed numbers of neurons, glial cells, muscles, and hypo-dermal cells of rigidly specified fates. These lineages reveal the ancestral relationships among specific cells of known structure and function; they thus complement the classical embryology, which defined the ancestral relationships among different organs. We have determined the postembryonic cell lineages by direct observation of living nematodes.
C. elegans is an excellent organism for the study of cell lineages. It is small, easily cultured, and readily amenable to genetic manipulations (Brenner, 1973, 1974). Like other nematodes (e.g., Chitwood and Chitwood, 1974), C. elegans is anatomically simple (Fig. 1). Its tubular body, consisting of a hypodermal wall and an underlying musculature, encloses its digestive and reproductive systems. Although it has most major differentiated tissue types (nerve, muscle, hypodermis, intestine, and gonad), C. elegans consists of relatively few cells; as we show below, the adult contains fewer than 1000 nongonadal nuclei.
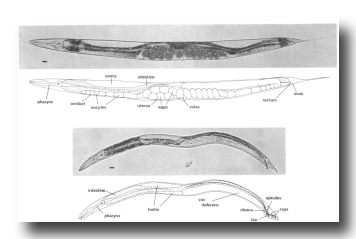 Figure 1. Adult hermaphrodite (above) and male (below), lateral views; bright field illumination. 137 x. Bar = 20 µm.
Figure 1. Adult hermaphrodite (above) and male (below), lateral views; bright field illumination. 137 x. Bar = 20 µm.
The life cycle of C. elegans is rapid; in 3.5 days (at 20°C) it develops from a fertilized egg through four larval stages to a mature adult. Superficially, the newly hatched larva appears to be quite similar to the adult. The most obvious developmental change is in the size and complexity of the gonad, which contains 4 nuclei in the young larva and increases to about 2500 in the mature adult (Hirsh et al., 1976). Gross morphological differences are also apparent in the nongonadal sexual structures of the hermaphrodite and male; these structures are not present in young larvae (Figs. 1 and 2).
Recently, one of us (Sulston, 1976) described a technique with which it is possible to determine cell lineages by observing living nematodes. When appropriately mounted on a thin block of agar on a microscope slide, a nematode proceeds through its normal life cycle. Observation of such animals under Nomarski differential interference contrast optics in the light microscope allows one to directly follow the migrations, divisions, and deaths of individual cells.
Using this technique, we have extended the earlier study (Sulston, 1976) of development in the ventral nervous system of C. elegans. In this paper, we report the complete postembryonic nongonadal cell lineages of C. elegans. These lineages produce the accessory sexual structures of the hermaphrodite and male and lead to other significant developmental changes in the neuronal, muscular, hypodermal, and digestive systems.
To provide a framework for describing the anatomical changes which occur during larval development, we begin with a detailed account of the cellular anatomy of the young nematode. This anatomy has been determined by a combination of Nomarski and electron microscopy.
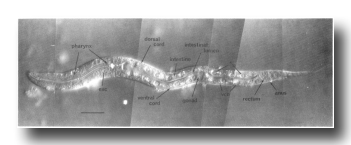 Figure 2. Young L1 hermaphrodite, lateral view; Nomarski optics. Montage of photographs of a single living animal. exc, excretory cell; i, intestinal nuclei; vcn, ventral cord neurons. Bar = 20 µm.
Figure 2. Young L1 hermaphrodite, lateral view; Nomarski optics. Montage of photographs of a single living animal. exc, excretory cell; i, intestinal nuclei; vcn, ventral cord neurons. Bar = 20 µm.
Readers who do not wish to become submerged in the detailed descriptions under Materials and Methods and Results will find a summary of our observations and conclusions under Discussion.
Materials & Methods
(A) Nematode Strains
Caenorhabditis elegans var. Bristol (strain N2) was obtained from Sydney Brenner and grown on Escherichia coli OP50 on petri dishes at 20°C, as described previously (Brenner, 1974). Hermaphrodite stocks were propagated by self-fertilization; male stocks were propagated by crossing with hermaphrodites.
Aphelencoides blastophthorus, Longidorus macrosoma, Panagrellus redivivus (Panagrellus silusiae), and Turbatrix aceti were obtained from David J. Hooper, Rothamsted Experimental Station, Har penden, Hertfordshire, England. Ascaris lumbricoides var. suis was obtained from the local slaughterhouse; eggs were squeezed out of adult uteri and development was initiated by incubation in 0.1 M sulfuric acid at 25°C (Rogers, 1960); at appropriate intervals, samples were washed with insect tissue culture medium (Shields et al., 1975) and larvae were released from the eggs by gently rolling the tip of a Pasteur pipet over them.
(B) Techniques
(1) Study of Living Specimens
(a) Mounting. An agar slab about 0.5 mm thick was prepared by flattening a drop of 5% agar on a microscope slide with a second, siliconized slide placed across it; the siliconized slide was supported by "spacer" slides raised by one or two thicknesses of adhesive tape. After the agar had hardened, the siliconized slide was removed and a small drop (about 2 microlitre) of 10% polyvinylpyrrolidone (molecular weight 44,000; BDH Chemicals, Ltd., Poole, England) in S medium (Sulston and Brenner, 1974) was placed on the agar, care being taken not to break its surface. A selected nematode was transferred to this drop, using either a sharpened wooden stick or a human eyelash attached with Plasticine to a stick. An animal successfully transferred would thrash wildly. The center of a 12 x 12-mm coverslip was very thinly coated with E. coli OP50 scraped from a petri dish with a wire loop. The coverslip was lowered gently onto the agar, care again being taken not to break the agar surface. The volume of liquid was such that the pressure of the coverslip prevented the worm from thrashing without excessively inhibiting its movement. All of these operations were performed at a room temperature of about 20°C.
Excess agar protruding beyond the coverslip was removed with a razor blade. The edges of the remaining agar block were sealed with silicone grease or Vaseline to prevent dehydration. Air bubbles which formed when the coverslip was positioned were gradually absorbed by the agar. After a period of quiescence (less than 1 hr), the nematode generally moved into the bacterial lawn and started to feed.
Nematodes mounted for observation in the light microscope could be safely removed afterward for other types of study (e.g., electron microscopy).
(b) Observation. Nematodes were observed in either a Zeiss Universal or a Zeiss Standard RA microscope equipped with a Plan 100 objective and Nomarski differential interference contrast optics. To reduce heating, illumination was kept as low as possible; a heat filter was routinely used and, occasionally, a broadband green interference filter was also employed. Air temperature was maintained between 19 and 22°C.
Because C. elegans is small and transparent, internal structures in intact living specimens could be readily examined. Nuclei and large nucleoli were clearly resolved by Nomarski optics, which permits visualization of changes in refractive index and has a very shallow depth of field (e.g., Fig 2). Cell boundaries, however, were not always visible. The nuclei of different types of cells were generally distinguishable and are described under Results.
This technique is nondestructive and allows the nematode to live under reasonably natural conditions. While mounted for observation, the nematode moved freely between the coverslip and the surface of the agar block. Attracted by the bacterial lawn, it generally stayed near the middle of the coverslip. If the nematode strayed out from under the coverslip, it could be transferred to a petri dish and remounted.
C. elegans can only flex in a dorso-ventral direction. Hence, because it was restricted to the plane between the agar and the coverslip, the nematode was observed lying on its side. A properly mounted animal usually remained lying on the same side. However, for a period just before molting, nematodes actively and repeatedly invert their positions with a rapid twisting movement. On occasion, such "flips" proved very inconvenient, as they made it difficult to continue observing cells which had moved from the side nearest the coverslip to the side nearest the agar slab (because of interference introduced by the body of the nematode).
(c) Cell lineages. Lineages were determined by continually observing given nuclei as they migrated, divided, and died during the course of postembryonic development. Because cell boundaries were often not visible, these lineages depict the behavior of nuclei and not necessarily the behavior of cells; it is possible that some nuclear divisions (or movements) are not accompanied by concomitant cellular divisions (or movements). During periods of nuclear activity, sketches of those nuclei of interest and of appropriate adjacent landmarks (usually other nuclei) were made as frequently as possible. During periods of nuclear quiescence, observations were recorded at intervals of 0.5-2 hr. When lineages in a given nematode were to be followed for more than 1 day, the animal was refrigerated overnight at 6-8°C during a suitable quiescent period. Nematodes were generally chilled during the early period of a given larval stage. (Individuals refrigerated just prior to or during lethargus —a 2-hr period preceding each of the four larval molts during which there is little pharyngeal pumping or body movement — often did not recover.) When returned to 20°C, most individuals recommenced development after a 0.5- to 2-hr lag. Generally, nematodes were refrigerated for no more than 15 hr.
When appropriately mounted, healthy nematodes developed as rapidly as on petri dishes. Some individuals, particularly after refrigeration, developed more slowly. Occasionally, a younger worm was damaged during the mounting process and barely developed at all; such individuals were discarded. To obtain a standard time scale for the lineage charts presented below, observed time intervals were normalized according to the observed length of the appropriate intermolt period.
On the lineage charts, the time of a molt is defined as the moment when the head of the nematode breaks through the old cuticle after lethargus. Lethargus is indicated by dotted regions along the time scales. The time of a cell division reflects the time a metaphase plate is visible (see Results, Cell Division). The time of a cell death indicates the time of maximal nuclear refractility (see Results, Programmed Cell Death).
(d) Photography. Photomicrographs were taken with a Zeiss microflash illuminator. All photomicrographs are of living specimens.
(2) Cell Assignments
Nuclei observed with Nomarski optics were assigned to specific cell types by tracing them through to the adult stage, in which they have been identified by reconstruction from serial section electron micrographs available in this laboratory and prepared as described by Ward et al. (1975).
(3) Camera Lucida Drawings
Drawings were made using a Zeiss camera lucida. Specimens were mounted on slabs of 5% agar as described above, except (1) no bacteria were added, and (2) the molten agar contained l-phenoxy-2-propanol (Bird, 1971) as an anesthetic. The concentrations of phenoxypropanol used were 0.2% v:v for L1's, 0.3% for L2's and L3's, and 0.5% for L4's and adults. (Ln denotes animals of the nth larval stage.)
The line drawings of nematodes presented below are based upon camera lucida drawings. They show nuclei, nucleoli (when visible), and other appropriate morphological landmarks. These drawings depict typical individuals, but, as there are minor variations from animal to animal, they cannot always be used to identify nuclei in animals in which lineages have not been traced directly. Also, minor distortions in nuclear position sometimes are evident in anesthetized nematodes.
(4) Feulgen Staining
A small drop of 10% ovalbumin was placed on a glass slide coated with a thin layer of collagen prepared according to Bornstein (1958). A large number of nematodes were spread evenly over the surface of the slide with a paper strip; alternatively, single worms were transferred with a wooden stick or platinum wire. When necessary to prevent evaporation, the slide was kept cool over ice. The slide was placed in Carnoy's fixative (Pearse, 1968) for at least 1 hr; overnight fixation was preferable. After rehydration through 50 and 30% ethanol (10 min each), the slide was incubated in 1 N HCl at room temperature for 10-20 min and then in 1N HCl at 60°C for 10-12 min. It was transferred to Schiff's reagent for 1.5-2 hr. Schiff's reagent was prepared by bubbling sulfur dioxide gas through 1% basic fuchsin, essentially as described by Rafalko (1946). After staining, the slide was rinsed twice in distilled water and dehydrated through a graded series of alcohols (30, 50, 70, and 90%, 10 min each). After two 30-min baths in absolute ethanol and two 30-min baths in xylene, the slide was mounted in Depex mounting medium (Gurrs, London, England). Stained specimens were examined using bright field illumination through a blue filter.
(5) Laser
A coumarin dye laser microbeam system developed by J. G. White was used to kill specific cells in individual nematodes. This system produces a focused spot about 1.5 micrometer in diameter and can destroy a given cell with no apparent damage to its neighbors. It is based upon a system developed by Berns (1972) and will be described in a future publication by Dr. White.
(C) Nomenclature
In describing the anatomy and development of C. elegans, we have adopted the following system to name specific cells (or nuclei) and their daughters. Upper-case letters indicate blast cells of unknown embryonic lineage; for example, M is a mesoblast present in the newly hatched L1. In some cases, a set of blast cells undergoes similar patterns of cleavage and produces morphologically similar groups of daughters; each member of such a set is denoted with a common upper-case letter followed by a specific numeric designation, e.g., six blast cells on each side of the lateral hypodermis are V1-V6. When a blast cell divides, each daughter is named by adding to the name of its mother cell a single lower-case letter representing its position immediately after division relative to its sister cell. A cell which divides along a dorso-ventral axis has its daughters indicated by a "d" and a "v"; e.g., M divides to produce M.d and M.v. Left-right divisions are indicated by "l" and "r"; e.g., M.d divides to produce M.dl and M.dr. Anterior-posterior divisions are indicated by an "a" and a "p"; e.g., M.dl divides to produce M.dla and M.dlp. As indicated in these examples, a period separates the name of the original blast cell from the labels which define specific progeny of subsequent divisions.
Within the lineage trees, "d," "l," and "a" daughters are represented by left branches, and "v," "r," and "p" daughters are represented by right branches; these branches are labeled accordingly (except in lineages with most divisions anterior-posterior, in which the labels a and p are omitted from the branches). An oblique division is indicated on the branches by labels with two (or three) characters; the first character from these labels defines the branch assignments. For example, E.r divides along an anterior/ventral-posterior/dorsal axis; hence, the left branch is labeled "av," and the right branch is labeled "pd" (Fig. 22). The daughters of such a division are named by using only the first of the characters which indicate the division axis, i.e., E.ra and E.rp. Thus, the number of characters which follow a blast cell name directly implies the number of divisions which generated the progeny cell; e.g., E.rap was produced by the third division of E.
In the B lineage, the precise fates of certain progeny cells are not predetermined. For example, B.alaa and B.araa, generated on the left and right sides respectively, recentralize. Their relative anterior-posterior position after recentralization is indeterminate. The anterior cell ("Bα") follows one lineage program, whereas the posterior cell ("Bβ") follows another. In other words, either B.alaa or B.araa can become Bα. Thus a greek letter following a blast cell name indicates a cell derived from that blast cell via one of a number of alternative lineage routes.
Entirely lower-case names are used as abbreviations for cell types in lineage charts, camera lucida drawings, and photographs. For example, "bm" identifies a body muscle. In the lineage charts, this name follows the systematic name based on the lineage history of the cell, e.g., M.dlpp; bm. The abbreviations used are: bm, body muscle; cc, coelomocyte; dep, anal depressor muscle; exc, excretory cell; exc gl, excretory gland; g, neuron or glial cell; hsn, hermaphrodite-specific neuron; i, intestinal nucleus; im, intestinal muscle; pig, posterior lateral ganglion; rect gl, rectal gland; se, seam cell; set, tail seam cell; sph, anal sphincter muscle; sy, syncytial nucleus; um1, type 1 uterine muscle; um2, type 2 uterine muscle; vcn, ventral cord neuron; vh, ventral hypodermal nucleus; vm1, type 1 vulval muscle; vm2, type 2 vulval muscle.
The nomenclature used in this paper is somewhat different from that used previously (Sulston, 1976) to describe the cell lineages of the ventral nervous system of C. elegans. We believe that the revised nomenclature is easier to use in referring to the relatively large number of cells described in this paper.
Results
(A) Anatomy
(1) Gross Morphology
(2) Newly Hatched Hermaphrodite
(a) Ectoderm
(b) Mesoderm
(c) Intestine
(d) Gonad
(e) Pharynx
(3) Newly Hatched Male
(B) Cell Lineages
(1) Cell Division
(2) Programmed Cell Death
(3) Development
(a) Ectoderm
(b) Mesoderm
(c) Intestine
(d) Gonad
(4) Other Nematodes
(A) Anatomy
(1) Gross Morphology
Like other nematodes (e.g., Chitwood and Chitwood, 1974), C. elegans has an elongated cylindrical body with tapered ends (figure 1 and figure 2). An external cuticle covers the hypodermal body wall. Beneath the hypodermis are four longitudinal rows of body muscles located subventrally and subdorsally. The mouth leads into the bilobed muscular pharynx, which pumps food into the tubular intestine to the rectum and anus. The nervous system consists of a circumpharyngeal nerve ring, dorsal and ventral nerve cords, and a variety of sensory receptors and ganglia.
The reproductive system of the adult hermaphrodite produces both sperm and oocytes. Two reflexed gonadal arms, each of which contains an ovary, oviduct, spermatheca, and uterus, terminate at the vulva, located midway along the ventral side. The reproductive system of the adult male consists of a single testis which empties into the vas deferens; the vas deferens unites posteriorly with the rectum, forming a cloaca. Accessory sexual structures are located in the male tail.
(2) Newly Hatched Hermaphrodite
(a) Ectoderm. The ectoderm, which comprises the majority of cells in the L1, can be broadly divided into the nervous system with its supporting cells and the hypodermis. Embedded in the hypodermis are various blast cells and a number of cells of specialized functions.
The hypodermis essentially consists of four longitudinal ridges (dorsal, ventral, lateral right, and lateral left) joined circumferentially by thin sheets of cytoplasm which separate the muscle cells from the cuticle. Hypodermal nuclei, generally large and flat with large nucleoli (figure 3), are located in these four ridges. The dorsal ridge, which is filled with refractile granules in the late embryo and the young L1, normally contains nuclei only in the head. The ventral ridge contains hypodermal nuclei in the head and tail but not along the body in the young L1; as described below (see Cell Lineages, Ectoderm), a number of hypodermal nuclei are produced in the ventral ridge during later development. The lateral hypodermal ridges are almost bilaterally symmetrical and contain nuclei throughout their lengths. Twenty-four of these nuclei are arranged in six similar ventro-lateral pairs located on each side along the body; each pair includes one hypodermal precursor (V) and one precursor (P) of the ventral nervous system (figure 4 and figure 9). Anterior to the first of these pairs on each side there are two hypodermal precursors (H1 and H2). Each side of the tail has a single precursor (T). There are 11 other hypodermal nuclei on each side of the nematode. These nuclei are not bilaterally symmetrical in location; the set on the left appears to be displaced posteriorly relative to that on the right. Except for the precursor cells, almost all of the lateral hypodermal nuclei lie in a single syncytium (White, 1974). Some syncytial hypodermal nuclei do not occupy rigidly defined positions and seem to be moved passively during development.
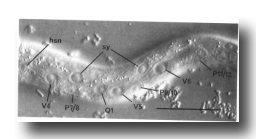 Figure 3. Left lateral hypodermis, posterior region of young L1 hermaphrodite, lateral view; Nomarski optics, hsn, hermaphrodite-specific neuron; sy, syncytial hypodermal nuclei. Bar = 20 µm.
Figure 3. Left lateral hypodermis, posterior region of young L1 hermaphrodite, lateral view; Nomarski optics, hsn, hermaphrodite-specific neuron; sy, syncytial hypodermal nuclei. Bar = 20 µm.
Three gland cells (rect gl) (Chitwood and Chitwood, 1974) ring the junction between the intestine and the rectum (figure 4). Two other cells (B and F) are located dorsal to the rectum; these cells divide only in the male. Another pair of cells is located laterally; the cell on the left (K) divides in both hermaphrodites and males. Two additional cells (C and E) are located ventral to the rectum and posterior to the preanal ganglion. C lies more ventrally and further right than E. Both C and E divide only in the male.
The cells of the so-called excretory system (Bird, 1971) lie in close association with the hypodermis. The nucleus of the 'H"-shaped excretory cell (exc) lies just left of the midline, ventral to the rear bulb of the pharynx. Immediately anterior to it is the nucleus of the duct cell. Two gland cells (exc gl) which feed into the excretory duct are located just posterior to the posterior-most region of the ventral ganglion. Although normally quiescent, the duct pulsates in dauer larvae. [The dauer larva is a developmental variant specialized for dispersal and/or survival in harsh conditions (Cassada and Russell, 1975)]. The rate of excretory duct pulsation in dauer larvae of other nematodes depends upon the osmolarity of the medium (e.g., Weinstein, 1952), indicating that one function of the "excretory" system is osmoregulation.
In the L1, the nuclei of the nervous system are generally distinct morphologically from those of the hypodermis. Neurons and glial cell nuclei are small and granular with no visible nucleoli (figure 2, figure 7, and figure 8); in older larvae and adults, these nuclei often acquire visible nucleoli. The adult anterior sensory nervous system has been described in detail (Ward et al., 1975; Ware et al., 1975); we have observed no postembryonic cell divisions in this system. Its cell bodies lie in groups anterior and posterior to the ring of nerve fibers which runs circumferentially around the isthmus of the pharynx. The posterior groups, known as the lateral ganglia, also include some interneuron and motoneuron cell bodies. In the young L1, the amphid sheath cell bodies (Ward et al., 1975) lie behind the pharynx and are embedded in the hypodermis; during larval development, they gradually move anteriorly and medially (figure 4 and figure 9). The ventral ganglion contains two blast cells (G1 and G2) and lies ventral and posterior to the nerve ring, extending to the excretory glands. The sublateral ganglia lie dorsolateral to the excretory glands. The retrovesicular ganglion, with 12 mature nuclei and 1 neuroblast (P0.a), lies ventral and posterior to the excretory cell and merges with the ventral nerve cord. The ventral nerve cord is the major longitudinal fiber bundle in the nematode and runs along the right side of the ventral hypodermal ridge; the dorsal nerve cord contains fewer fibers and runs along the left side of the dorsal hypodermal ridge. In the L1, 15 motoneuron cell bodies are present in the ventral cord. At the posterior end of the ventral cord is the pre-anal ganglion, which contains six nuclei. Two lumbar ganglia are present in the post-anal region; the ganglion on the left contains eight nuclei, and that on the right contains nine nuclei. A small dorso-rectal ganglion with two nuclei is also present in the tail.
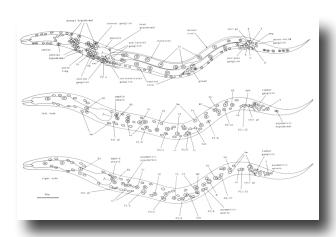 Figure 4. Young L1 hermaphrodite, lateral views, showing nuclei as seen in central, left, and right optical sections. Not all nuclei in the head ganglia are shown. These figures are somewhat schematic, as nuclei are not arranged precisely into three planes. Left-right pairs of P cells (e.g., Pl/2) are not finally named until after their subsequent migration into the ventral cord (see Cell Lineages, Development). bm, body muscle; cc, coelomocyte; dep, anal depressor muscle; exc, excretory cell; exc gl, excretory gland; hsn, hermaphrodite-specific neuron; im, intestinal muscle; rect gl, rectal gland; sph, anal sphincter muscle; sy, syncytial hypodermal nuclei.
Figure 4. Young L1 hermaphrodite, lateral views, showing nuclei as seen in central, left, and right optical sections. Not all nuclei in the head ganglia are shown. These figures are somewhat schematic, as nuclei are not arranged precisely into three planes. Left-right pairs of P cells (e.g., Pl/2) are not finally named until after their subsequent migration into the ventral cord (see Cell Lineages, Development). bm, body muscle; cc, coelomocyte; dep, anal depressor muscle; exc, excretory cell; exc gl, excretory gland; hsn, hermaphrodite-specific neuron; im, intestinal muscle; rect gl, rectal gland; sph, anal sphincter muscle; sy, syncytial hypodermal nuclei.
On each lateral side of the nematode, four isolated cell bodies with neuronal-like nuclei are embedded in the hypodermis immediately beneath the cuticle (figure 4 and figure 9). The small subventral neuron (hsn) near the gonad primordium in the hermaphrodite is absent in the male (figure 5). Two other lateral neuronal-like cells are located (a) just anterior to V1 and (b) subdorsally, near V3. A fourth nucleus with similar morphology is present between V3 and V4, anterior to the hermaphrodite-specific neuron; although this nucleus becomes more hypodermal-like during development, the cell nonetheless appears to be a neuron or supporting cell in the adult.
Also lying subdorsally in the lateral hypodermis between V4 and V5 is a pair of neuroblasts: one on the left side (Q1) and one on the right side (Q2).
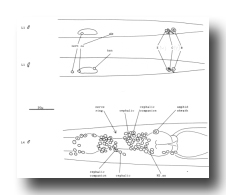 Figure 5. Anatomical differences between the young hermaphrodite and the young male. The top two figures show lateral views of the posterior halves of a young L1 male and hermaphrodite. The sex-specific characteristics shown are (a) position of a left coelomocyte (cc), (b) presence of hermaphrodite-specific neurons (hsn), and (c) size of the B and C nuclei. Four male-specific cephalic companion neurons, also present at hatching, are best seen during the L4 stage, as shown in the bottom figure (left lateral view). The position of H2.aa in the lateral ganglion is indicated.
Figure 5. Anatomical differences between the young hermaphrodite and the young male. The top two figures show lateral views of the posterior halves of a young L1 male and hermaphrodite. The sex-specific characteristics shown are (a) position of a left coelomocyte (cc), (b) presence of hermaphrodite-specific neurons (hsn), and (c) size of the B and C nuclei. Four male-specific cephalic companion neurons, also present at hatching, are best seen during the L4 stage, as shown in the bottom figure (left lateral view). The position of H2.aa in the lateral ganglion is indicated.
(b) Mesoderm. At hatching, the L1 contains 81 body muscle cells (bm) which control its locomotion. The nuclei of these cells are ovoid with a granular nucleoplasm surrounding a spherical nucleolus (figure 6). During the L2 stage, the nucleoplasm becomes smooth and remains so throughout the rest of development. The somatic muscle nuclei are distributed almost symmetrically in four longitudinal rows located subventrally and subdorsally (figure 4 and figure 23). Each dorsal quadrant contains 21 somatic muscle cells, the ventral right quadrant contains 20, and the ventral left quadrant contains 19. The ventral asymmetry appears to result from a gap on the left side somewhat posterior to the gonad primordial.
Four specialized muscle cells are present in the body of the newly hatched larva Two are intestinal muscles (im). These muscles run subventrally along the posterior-most region of the intestine and attach to it and to the thin layer of hypodermis just ventral to the lateral cords; their filaments are oriented longitudinally. The intestinal muscle nuclei are located slightly dorso-laterally to the ventral body muscle rows and are morphologically similar to but somewhat smaller than body muscle nuclei. Slightly posterior to these cells is a single saddle-shaped muscle (sph), which almost surrounds the posterior end of the intestine and probably functions as a sphincter muscle to control defecation. The nucleus of this sphincter muscle is located somewhat left of the central midline. An H-shaped "anal depressor" muscle cell (dep), with its nucleus located centrally in the crossbar of the H, is located posterior to the anus and opens the anus during defecation.
A single mesodermal blast cell (M) is present on the right side of the young L1 somewhat posterior to the gonad primordium (figure 6). This cell has a large, relatively flat nucleus and a large nucleolus.
Four coelomocytes (cc) (Chitwood and Chitwood, 1974) are also present in the L1. These glandular cells are located in the pseudocoelom adjacent to the somatic musculature. In the young L1, their nuclei are granulated and do not contain visible nucleoli (figure 6). During larval development, the cytoplasm of the coelomocytes acquires both granules (of high refractive index) and vacuoles (of low refractive index), giving these cells a very characteristic appearance (figure 6). In the L1 hermaphrodite, all four coelomocytes are located sub-ventrally between the pharynx and the gonad primordium; the two on the right are anterior to the two on the left (figure 4 and figure 23). In the male, one of the left coelomocytes is located posterior to the gonad primordium (figure 5). We consider these coelomocytes to be mesodermal in origin because, as described below (see Cell Lineages, Development), other coelomocytes are generated by blast cells which produce muscles during larval development.
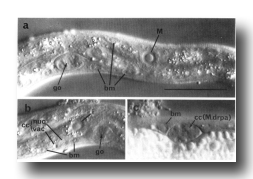 Figure 6. Plate 3 - Representative section through the isthmus.
Three gland cell ducts (d.) are present here. Symbols as in table 1. (Magn. x 9300.)
Figure 6. Plate 3 - Representative section through the isthmus.
Three gland cell ducts (d.) are present here. Symbols as in table 1. (Magn. x 9300.)
Located centrally, dorsal to the rear bulb of the pharynx, is a single cell with a muscle-like nucleus and a nucleolus slightly smaller than that of a body muscle. This cell is often squashed in appearance. Electron micrographs reveal that this head mesodermal cell forms gap junctions (White et al., 1976) with adjacent body muscles.
(c) Intestine. The intestine of the L1 is a hollow tube which normally contains 20 large, round nuclei with large nucleoli. (figure 2); its cytoplasm is usually filled with refractile granules. Along most of the length of the intestine, nuclei are located dorsal and ventral to the lumen; in the anterior-most region, however, a ring of four nuclei surrounds the lumen. This anterior-most region has a thinner internal boundary than the rest of the intestine; electron micrographs reveal that the microvilli in this region are approximately half the length of those found elsewhere. The number of intestinal nuclei is not rigidly determined, as individuals with 19, 21, and 22 nuclei have been observed. The intestine has a left-handed twist of 180° about the longitudinal axis of the nematode in the region of the primordial gonad. As such a twist can introduce a left-handed superhelical twist, we suspect that the bilateral asymmetry of the position of the intestine -its anterior part is displaced slightly left of the midline, and its posterior part, slightly right -is caused by this rotational twist.
Pale at hatching, the cytoplasm of the intestine becomes filled with refractile droplets as the nematode feeds. Lethargus (or starvation), causes the intestine to again become relatively pale. Subsequent feeding restores the refractile droplets, which are therefore probably involved in energy storage. Because of the opacity of the well-fed intestine, hypodermal nuclei on the lower side of the nematode are difficult to observe between molts.
(d) Gonad. The gonad primordium consists of four cells in the young L1 (figure 4). The two larger cells are adjacent; the two smaller cells are slightly anterior and posterior to them, respectively. All four nuclei have relatively large nucleoli (figure 2). The gonad primordium lies obliquely, with its anterior cells located slightly right and its posterior ones located slightly left. This bilaterally asymmetric positioning is probably related to the twist in the intestine (see above, Intestine). The gonad primordium of the male is morphologically identical to that of the hermaphrodite.
(e) Pharynx. The morphology of the pharynx of the adult hermaphrodite has recently been described in detail (Albertson and Thomson, 1976). No postembryonic cell divisions or deaths have been seen in the pharynx.
(3) Newly Hatched Male
At hatching, the L1 male is very similar in structure to the L1 hermaphrodite. We have found three criteria by which the sex of a young nematode can be determined (figure 5): (1) In the male, a left coelomocyte lies posterior to the gonad primordium. (2) The male is missing a neuronal-like nucleus embedded in each side of the lateral hypodermis near the gonad primordium. (3) Ectodermal nuclei B and C near the anus enlarge soon after hatching in the male; these nuclei divide only in the male.
In addition, the male head contains four neuronal-like nuclei which are absent in the hermaphrodite. These nuclei are located close to the cell bodies of the cephalic neurons and presumably are the cell bodies of the four male-specific sensory processes in the cephalic sensilla discovered by Ward et al. (1975). Although present in the newly hatched larva, these "cephalic companion" cell nuclei are more easily distinguished in the L4, in which they have relatively large distinct nucleoli (figure 5), possibly reflecting increased activity at this stage of development.
(B) Cell Lineages
(1) Cell Division
Although the size and shape of different blast cells vary, the overall process of cell division as observed with Nomarski optics during the postembryonic development of C. elegans is quite uniform (figure 7). For several hours before division, the nucleus of a blast cell generally has a prominent nucleolus and a smooth nucleoplasm. About 10 min before division, the nucleoplasm becomes coarsely granular, often producing a "rosette" around the nucleolus. In rapid succession during the next few minutes, the nucleolus disappears, the nuclear membrane breaks down, and the metaphase plate forms. During early anaphase, the plate splits and the two halves begin to move toward the poles; individual chromosomes are usually not visible. For a short time, nuclear components cannot be seen; the daughter nuclei then gradually appear. Finally, in some cells, daughter nucleoli are formed.
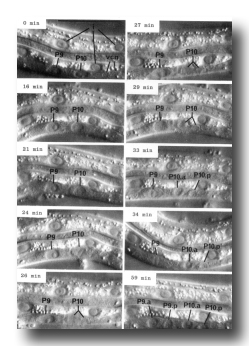 Figure 7. Cell division. Sequential photographs of an L1 hermaphrodite, lateral view; Nomarski optics vcn, ventral cord neurons. 0 min, interphase; 16 and 21 min, P10 prophase; 24 min, P10 metaphase; 26 min 10 anaphase; 27 mm, P10 telophase; 29 min, P9 prophase; 33 and 34 min, P9 metaphase. Bar = 20 µm.
Figure 7. Cell division. Sequential photographs of an L1 hermaphrodite, lateral view; Nomarski optics vcn, ventral cord neurons. 0 min, interphase; 16 and 21 min, P10 prophase; 24 min, P10 metaphase; 26 min 10 anaphase; 27 mm, P10 telophase; 29 min, P9 prophase; 33 and 34 min, P9 metaphase. Bar = 20 µm.
(2) Programmed Cell Death.
In a number of the ectodermal cell lineages, certain cells undergo a series of morphological changes which we interpret as programmed cell death (Saunders, 1966) (figure 8). Blobs appear at the perimeter of the nucleus. The nucleoplasm becomes less granular and somewhat more refractile. There is then a marked increase in the refractility of the nucleus, giving it a characteristic flattened appearance. The internal refractility diminishes slightly, leaving a refractile shell at the surface of the nucleus. The nucleus shrinks and it gradually disappears. Generally, other nuclei completely obliterate the site, although in some cases a permanent gap remains. Either way, no subsequent trace of the dead cell can be detected either by Feulgen staining or by electron microscopy.
Most cell deaths are of posterior daughters from anterio-posterior divisions. As described below (see Cell Lineages), the pattern of cell deaths in the male differs from that in the hermaphrodite.
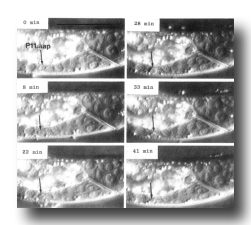 Figure 8. Cell death. Sequential photographs of an L1 hermaphrodite, lateral view; Nomarski optics. The arrow points to the dying cell, P11.aap. Bar = 20 µm.
Figure 8. Cell death. Sequential photographs of an L1 hermaphrodite, lateral view; Nomarski optics. The arrow points to the dying cell, P11.aap. Bar = 20 µm.
(3) Development
To the best of our knowledge, the results described in this section include all non-gonadal cell divisions and deaths that occur during the postembryonic development of C. elegans. However, very late developmental events in the male tail could have been missed. Because the detailed anatomy of the adult male tail is not known, many of its nuclei seen with the light microscope could not be assigned to specific cell types.
Cell lineages not specifically described for the male are identical to those which occur in the hermaphrodite.
(a) Ectoderm
V cells, hermaphrodite (figure 9 and figure 10): All 12 of the ventrolateral hypodermal precursor cells (V) divide about midway through the L1 stage. The anterior daughters enter the hypodermal syncytium. The posterior daughters remain separate from the syncytium; together with one head hypodermal cell (on each side) present at hatching, the posterior daughters form a distinct row of cells embedded in each lateral hypodermal ridge. Such cells have been named "seam" cells. Seam cells display a characteristic periodic activity. Toward the end of each intermolt period, the cytoplasm of the seam cells fills with refractile blobs (figure 11). This activity continues until midway through lethargus. That this activity is seen prior to the L4 molt (when the seam cells do not divide; see below) as well as prior to the other three molts (when the seam cells do divide) suggests that it is related to molting rather than to cell division.
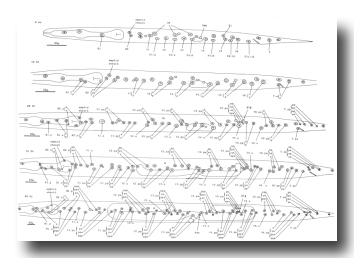 Figure 9. H, V, and T.a; hermaphrodite, lateral views; development of the left lateral hypodermis. Seam cells are indicated by dotted lines. hsn, hermaphrodite-specific neuron; plg, posterior lateral ganglion (see colorized version)
Figure 9. H, V, and T.a; hermaphrodite, lateral views; development of the left lateral hypodermis. Seam cells are indicated by dotted lines. hsn, hermaphrodite-specific neuron; plg, posterior lateral ganglion (see colorized version)
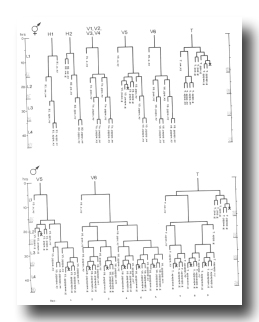 Figure 10. H, V, and T lineages, hermaphrodite and male; development of the lateral hypodermis. All divisions are anterior-posterior. g, neuron or glial cell; se, seam; set, tail seam (a separate group of cells which shows seam-like cytoplasmic activity in the late L4 male); sy, syncytial.
Figure 10. H, V, and T lineages, hermaphrodite and male; development of the lateral hypodermis. All divisions are anterior-posterior. g, neuron or glial cell; se, seam; set, tail seam (a separate group of cells which shows seam-like cytoplasmic activity in the late L4 male); sy, syncytial.
 Figure 11. Seam cell. Cytoplasmic activity in H1.aa of late L4 hermaphrodite, lateral view; Nomarski optics. The seam is outlined with a dotted line. Bar = 20 µm.
Figure 11. Seam cell. Cytoplasmic activity in H1.aa of late L4 hermaphrodite, lateral view; Nomarski optics. The seam is outlined with a dotted line. Bar = 20 µm.
The Vn.p seam cells undergo two rounds of division at about the time of the L1 molt; each produces a longitudinal row of four small nuclei. With the exception of V5.pap, the posterior daughters of the second round of divisions are themselves seam cells that divide at the L2 and L3 molts. The posterior daughter of each of these divisions is itself a seam cell. All of the other hypodermal progeny nuclei become part of the syncytium. In the L4 and adult, the seam cells form a continuous lateral band.
During the L2 stage, the lineage of V5 becomes distinct from that of the other V cells. On each side of the nematode, V5.paa and V5.pap undergo a series of divisions to produce four daughter nuclei. Together with two other nuclei on the left side (Q1.paa and Q1.pap; see below, Q Cells), these nuclei compose the posterior lateral ganglia, which consist of six cells on the left side and four cells on the right side. Ultrastructural studies of the posterior lateral ganglia show that they are positioned beneath the "postdeirids" (Chitwood and Chitwood, 1974) and contain the cell bodies associated with these sensilla. The proposed homology (Chitwood and Chitwood, 1974) between the postdeirids and the deirids in the head is supported by the observation that each of these sensilla has a single neuron containing the neuro-transmitter dopamine (Sulston et al., 1975). We have now identified the dopaminergic cell in each posterior lateral ganglion as being V5.paaa. This assignment was suggested by correlating the formaldehyde-induced fluorescence of the dopaminergic cell with the position of the V5.paaa cell body as visualized by Feulgen staining, using methods described previously (Sulston et al., 1975); it was confirmed by killing selected cells of known lineage with a laser microbeam and showing that the death of V5.paaa or of any of its ancestors (but not of any other cell) leads to loss of the formaldehyde - induced fluorescence. These experiments failed to reveal any regulative capacity of the cells involved in the development of the posterior lateral ganglia; no extra divisions occurred to replace those cells destroyed with the laser.
H cells, hermaphrodite (figure 9 and figure 10): The lineages of the hypodermal precursors of the head (H1 and H2) appear to be related to those of the V cells. Both generate seam cells as well as hypodermal nuclei in the syncytium, and both utilize similar stem cell patterns of cell division. However, there is an inversion of the pattern of the divisions of H1 compared to that of the V cells; the anterior daughter H1.a is the blast cell of the H1 lineage, whereas the posterior daughter Vn.p is the blast cell of the V lineage. It should be noted that H1.a and H1.p slowly reverse their anterior-posterior positions; however, this positional reversal is probably not the cause of the inversion of the pattern of divisions, because H1.a has the morphological appearance of a blast cell before any movement of its nucleus occurs. H1.aa has the appearance of a seam cell; unlike the "equivalent" Vn.pa cells, however, H1.aa does not divide. The lineage of H2, which is related to those of H1 and the V cells, produces seam cells, syncytial hypodermal nuclei, and one neuron-like cell (H2.aa), which is positioned in the mass of cell bodies posterior to the nerve ring (figure 5 and figure 9). That H1 and H2 together produce only half the number of syncytial hypodermal nuclei expected from two V cells may relate to the fact that postembryonic growth of the head is much less than that of the body (Hodgkin, 1974).
T cell, hermaphrodite (figure 9, figure 10, and figure 12): The tail precursor-cell T generates both hypodermal and nerve cells. Its anterior daughter T.a produces a lineage initially like that of a V cell; however, this similarity in the pattern and timing of cell divisions is not reflected in the morphology of the progeny. In the neuronal lineage of T.p (figure 10 and figure 12), there are two anterior-posterior positional inversions. First, about 1 hr after it is formed, T.pp begins to move slowly past T.pa. This migration appears to be an active process; neighboring cells seem to hinder rather than assist the movement. Later, T.pppap moves anterior to its sister; its nucleus becomes considerably deformed as it squeezes past other cells to reach its final position.
The fates of the progeny of the T cells in the adult have not yet all been established; at least some of these progeny appear to be associated with the phasmids, post-anal sensilla located on each side of the nematode (Chitwood and Chitwood, 1974).
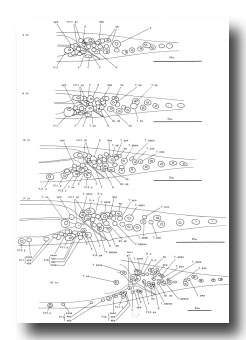 Figure 12. T, K, P11, and P12; hermaphrodite, left lateral views; development of the hermaphrodite tail. Only left and central nuclei are drawn.
Figure 12. T, K, P11, and P12; hermaphrodite, left lateral views; development of the hermaphrodite tail. Only left and central nuclei are drawn.
Q cells (figure 13 and figure 14): The left and right lateral neuroblasts, Q1 and Q2, display bilaterally symmetrical patterns of cell division. However, their progeny are not symmetrical in their movements: Q2.a, Q2.p, and Q2.ap all migrate anteriorly, while Q1.ap migrates posteriorly.
The fate of Q2.pp is variable. In hermaphrodites, it becomes less and less distinct and usually disappears, although in one individual it was observed to survive into the adult. In two males, Q2.pp underwent typical programmed cell death. Perhaps its fate is weakly sex specific.
 Figure 13. Q, left lateral views; development of the lateral neuroblasts. Sometimes, Q2.paa and Q2.pap move directly to their final positions, without circling around one another.
Figure 13. Q, left lateral views; development of the lateral neuroblasts. Sometimes, Q2.paa and Q2.pap move directly to their final positions, without circling around one another.
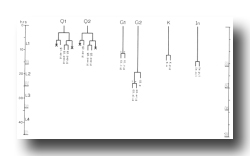 Figure 14. Q, G, K, and I lineages. Q1 and Q2 are lateral neuroblasts; G1 and G2 are in the ventral ganglion; K is in the tail; In are intestinal nuclei. Divisions are anterior-posterior unless otherwise indicated. g, neuron or glial cell; i, intestine.
Figure 14. Q, G, K, and I lineages. Q1 and Q2 are lateral neuroblasts; G1 and G2 are in the ventral ganglion; K is in the tail; In are intestinal nuclei. Divisions are anterior-posterior unless otherwise indicated. g, neuron or glial cell; i, intestine.
P cells, hermaphrodite (figure 12, figure 15, figure 16, figure 17, and figure 18): The P precursor cells are responsible for the postembryonic development of the ventral nerve cord and its associated ganglia. One of us has described the development of these systems in detail elsewhere (Sulston, 1976). For completeness, we summarize these observations below.
About the middle of the L1 stage, the ventro-lateral P cells appear to be "loosened" from their hypodermal neighbors. Cytoplasmic extensions of the P cells grow into the ventral cord. The nuclei of P1 and P2 migrate into these extensions; the residual cytoplasm soon follows (figure 15). After these movements of P1 and P2, similar migrations of successively more posterior pairs occur roughly in order along the cord; P11 and P12 migrate into the pre-anal ganglion.
There is some variability in the anterior-posterior order of a given left-right pair of P cells after they have entered the ventral cord; in at least some cases, the subsequent development of a cell depends not upon its side of origin, but rather upon its anterior-posterior position. For this reason, we number the P cells after their migration into the cord (figure 16). We have determined the extent of this variability for the following pairs. P1 generally arrives from the right side (in 14 out of 16 individuals examined), P11 arrives from the left (7 out of 7), and the entries of P3 and P5 are approximately random.
Soon after its migration, each P cell divides. All 12 P cells follow identical initial programs of division, each producing five neurons and one hypodermal cell (figure 17). In addition, a single neuroblast in the retrovesicular ganglion follows a program identical to that of the anterior daughters of the P cells (Pn.a); by analogy, we have named this neuroblast P0.a.
As cell divisions continue, the neuroblasts and their progeny move freely past the juvenile cells and the new hypodermal cells (Pn.p); the extent of this movement is variable, leading to different patterns of intercalation of new neurons with the juvenile and hypodermal cells in different individuals. Gradually, the ventral cord becomes evenly filled with nuclei. Toward the end of the period of division, the pattern of nerve cells is modified by a fixed program of cell deaths in the anterior and posterior regions of the cord. There is also an extra division of the most posterior hypodermal cell (P12.p), followed by the death of its posterior daughter (P12.pp).
Late during the L3 stage, the six ventral hypodermal nuclei (P3.p, P4.p, P5.p, P6.p, P7.p, P8.p) in close proximity to the developing gonad (figure 18) divide. The daughters of P5.p, P6.p, and P7.p move closer together and continue to divide to produce the 22 cells which form the vulva. The daughters of P3.p, P4.p, and P8.p become ventral hypodermal nuclei. In the adult, the ventral hypodermal nuclei formed during the development of the ventral cord lie in the hypodermal syncytium (White et al., 1976).

Figure 15. Ventral cord and muscle development. L1 hermaphrodite, left lateral view, central plane; Nomarski optics. The nucleus of P10 is just entering the cord. The intestine is pinched between M.d and M.v. Bar = µm.
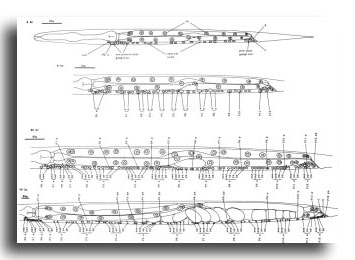 Figure 16. P and I; hermaphrodite, lateral views; development of the ventral nervous system and the intestine.
Figure 16. P and I; hermaphrodite, lateral views; development of the ventral nervous system and the intestine.
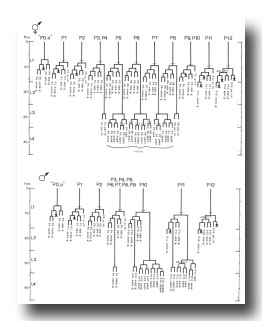 Figure 17. P lineages, hermaphrodite and male; development of the ventral nervous system. Dotted lines indicate the times nuclei migrate into the ventral cord. P0.a is so named for convenience; its actual ancestry is unknown. Divisions are anterior-posterior unless otherwise indicated. g, neuron or glial cell; vh, ventral hypodermal cell.
Figure 17. P lineages, hermaphrodite and male; development of the ventral nervous system. Dotted lines indicate the times nuclei migrate into the ventral cord. P0.a is so named for convenience; its actual ancestry is unknown. Divisions are anterior-posterior unless otherwise indicated. g, neuron or glial cell; vh, ventral hypodermal cell.
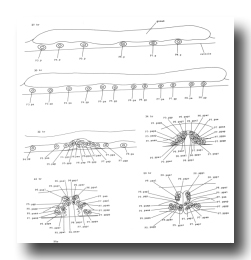 Figure 18. P3.p-P8.p; hermaphrodite, left lateral views midway along ventral side; development of the vulva.
Figure 18. P3.p-P8.p; hermaphrodite, left lateral views midway along ventral side; development of the vulva.
The fine-structure neuroanatomy of the ventral cord of the adult C. elegans hermaphrodite has been elucidated by White et al. (1976). The neurons in the ventral cord can be assigned to morphologically distinct classes which recur periodically along the length of the cord. The number of cells of each class is constant in different individuals, but slight variations in the relative positions of different classes occur. These anatomical observations can be explained by the developmental processes described above according to the following model. Each morphological class consists of those cells with an equivalent lineage history (Table 1); the positional variations result from the differential migrations which occur during development. The correlation of cell type with lineage history has been directly confirmed by using serial-section electron micrographs to reconstruct the retrovesicular ganglion and part of the ventral cord of individuals of known lineage (Table 1). However, three daughters located in the retrovesicular ganglion are not allocated as expected from their lineage history. As discussed below (see Discussion, Mechanisms of Determination), they may reflect end effects which seem to affect a number of aspects of the postembryonic development of C. elegans.
| (1) Morphological classes |
(2) Lineage history (model) |
(3) Confirmed by EM assignment |
| VA |
Pn.aaaa |
P2, P3, P4, P5, P6 |
| VB |
Pn.aaap |
P0, P1, P2, P3, P4, P5, P6 |
| VC |
Pn.aap |
P3, P4, P5, P6 DAS |
| DAS |
Pn.apa |
P1, P2, P3, P4, P5, P6 |
| VD |
Pn.app |
P0, P1, P2, P3, P4, P5, P6 |
| DA, DB, DD |
Juvenile |
Eight anterior-most juvenile cells of ventral cord (three anterior-most established in individuals of known lineage). |
Table 1. Correlation of morphological class with lineage history of motor neurons in the ventral nervous system of the adult hermaphrodite*. *Column 1 indicates morphological classes as defined by White et al. (1976). Column 2 shows the predicted lineage history of cells of each morphological class according to the model. Column 3 indicates neurons of each morphological class which, by their positions and neighbors, are consistent with having the lineage listed; to date, morphological assignments have been completed only for the anterior ventral cord (containing the progeny of P0.a-P6). Assignments for neurons in boldface in Column 3 have been established in individuals of known lineage. Three neurons located in the retrovesicular ganglion are not allocated as expected from this model: P0.aaaa and P1.aaaa are nonmotor neurons, and P0.apa is of morphological class VA. Three progeny (P0.aap, P1.aap, P2.aap) undergo programmed cell death and thus are not found in the adult. All electron microscopy was done by White et al. (1976).
G cells (figure 4 and figure 14): G1, located near the anterior end of the ventral ganglion, divides transversely late during the L1 stage to produce daughters with neuronal-like nuclei. Also in the ventral ganglion, G2 divides during the L2 stage. Like G1, G2.a then divides transversely and produces two daughters with neuronal-like nuclei.
K cell (figure 12 and figure 14): The solitary K precursor cell in the tail divides once about 3.5 hr before the L1 molt. K.p joins the two other neurons in the dorso-rectal ganglion. K.a is a hypodermal cell which completes a bilaterally symmetrical pair with a cell located right of the midline and present in the young L1.
Other cell deaths: On two occasions, other cell deaths were observed early during the L1 stage. One was immediately anterior to the excretory cell and the other was in the dorsal region of the lateral ganglion. These two cell deaths may normally be embryonic events which, in some individuals, are delayed until after hatching.
Male: Below, we first describe those ectodermal lineages which occur in the hermaphrodite and are modified in the male (V, T, P); we then describe male-specific lineages derived from cells present in the newly hatched L1 which do not divide in the hermaphrodite (B, F, C, E).
V and T cells, male (figure 10 and figure 19): The V and T lineages of the male are identical to those of the hermaphrodite until L2 lethargus; thus, the male carries all tail and posterior lateral cell bodies found in the hermaphrodite. Further divisions in the V5, V6, and T lineages then generate nine similar sets of neuronal-like nuclei on each side of the nematode as well as a number of larger hypodermal-like nuclei. Numerically, these nine sets correspond to the nine "rays" found on each side of the male tail (figure 1 and figure 20). These rays lie in a noncellular cuticular webbing, which forms a leaf-shaped fan. The entire structure, consisting of fan and rays, probably functions as a mechanosensory organ during copulation.
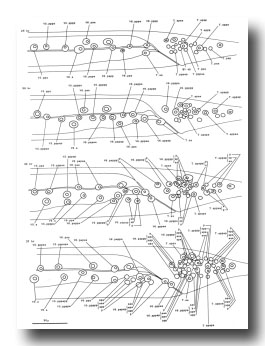 Figure 19. V5, V6, and T; male, left lateral views of the left side; development of the rays of the male tail. There is extensive rearrangement of the cells later, during L4 lethargus.
Figure 19. V5, V6, and T; male, left lateral views of the left side; development of the rays of the male tail. There is extensive rearrangement of the cells later, during L4 lethargus.
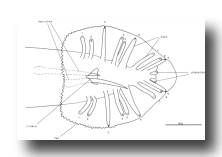 Figure 20. Male tail, ventral view. One spicule is extended and one is retracted. As indicated, the sensory tips of the rays lie either on the ventral (inside) surface (indicated by a solid circle) or on the dorsal (outside) surface (dotted circle).
Figure 20. Male tail, ventral view. One spicule is extended and one is retracted. As indicated, the sensory tips of the rays lie either on the ventral (inside) surface (indicated by a solid circle) or on the dorsal (outside) surface (dotted circle).
Preliminary experiments with the laser microbeam suggest how the V and T cell lineages lead to the development of the rays. Destruction of the precursor (e.g., V6.ppppp) of one of the nine sets of progeny cells with the laser results in the loss of a particular ray. In this way, the ancestry of the nine rays has been assigned (figure 10). Destruction of the precursor of ray 5, 7, or 9 also resulted in the loss of one of the three dopaminergic cells on that side of the male tail (Sulston et al., 1975). That only 6 of 18 morphologically similar rays have dopaminergic neurons suggests why mutant males lacking dopamine are still capable of mating (Sulston et al., 1975): at least some of the 12 other rays may act as alternative sensory elements.
The precise lineages of the six dopaminergic cells were then determined by destroying some of the progeny of the precursors of rays 5, 7, and 9. Usually, destruction of "aaa" (e.g., V6.pppppaaa) eliminated the dopaminergic cell, but did not eliminate the ray; destruction of "apa" (e.g., V6.pppppapa) eliminated neither the dopaminergic cell nor the ray; and destruction of "app" (e.g., V6.pppppapp) eliminated the ray, but did not eliminate the dopaminergic cell. These results suggest that, in each set, aaa is the neuron and app forms the structural element. Destruction of any one of the hypodermal nuclei "p" (e.g., V6.pppppp) associated with these sets produced no lesion detectable in the light microscope. As in the development of the posterior lateral ganglia (see above, V cells, hermaphrodite), laser experiments failed to reveal any potential for regulation.
P cells, male (figure 17 and figure 21): In the development of the male ventral nerve cord, there are fewer programmed cell deaths during the L1 stage and extra cell divisions during the L3 stage; both aspects of this "reprogramming" involve only the Pn.aap daughters. In these ways, the male produces 14 extra cells in the ventral cord.
In the male, ventral hypodermal nuclei P3.p-P8.p (which generate the vulva in the hermaphrodite) do not divide; instead, P10.p and P11.p divide to produce 16 male-specific nuclei in the region of the pre-anal ganglion. We do not know the fate of these nuclei in the adult male; preliminary observations suggest that some may be neurons. In 4 out of 17 individuals observed, P9.p divided once, yielding two ventral hypodermal nuclei.
Although 18 similar lineages generate 18 morphologically similar rays, lineally equivalent daughters do not all differentiate identically: Only six of the aaa progeny are dopaminergic. It will be interesting to determine if the other aaa progeny are indeed neurons and, if so, to identify what transmitter(s) they employ.
B and F cells, male (figure 21 and figure 22): Some of the descendants of the B and F cells form the cloaca; others produce the copulatory spicules, elongated cuticular structures used to open the vulva of the hermaphrodite during mating (figure 1 and figure 20). The specific fates of the daughters of these lineages have not been established. Most of these progeny cells are probably structural, as they have clear nucleoplasms with prominent nucleoli; a few, designated "g" in figure 22, are more compact with granular nucleoplasms and seem to be more like neurons or glial cells. However, unlike the neurons of the ventral cord and hermaphrodite tail, these cells often contain visible nucleoli soon after they are formed (possibly reflecting relatively rapid growth). Because of these ambiguities, any distinctions made between neurons and structural cells for these lineages are rather subjective.
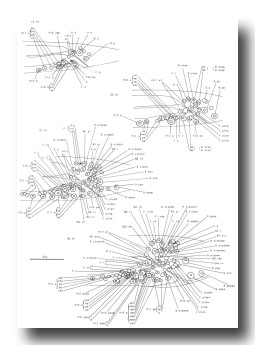 Figure 21. B, C, E, F, P10.p, and P11.p; male, lateral views; ectodermal development in the male tail. There is extensive rearrangement of the cells later, during L4 lethargus. Only left and central cells are shown.
Figure 21. B, C, E, F, P10.p, and P11.p; male, lateral views; ectodermal development in the male tail. There is extensive rearrangement of the cells later, during L4 lethargus. Only left and central cells are shown.
 Figure 22. B, C, E, and F lineages, male; ectodermal development in the male tail. g, neuron or glial cell.
Figure 22. B, C, E, and F lineages, male; ectodermal development in the male tail. g, neuron or glial cell.
B divides obliquely late during the L1 stage. During the L2 stage, its anterior-dorsal daughter (B.a) divides transversely; B.al and B.ar divide twice each, producing a row of four nuclei on the right and left sides of the tail. Both the anterior-most (B.alaa and B.araa) and posterior-most (B.alpp and B.arpp) of these nuclei recentralize. One member of each of these pairs becomes located anterior to the other, but there is no fixed pattern as to the order these cells assume; all four possible final configurations have been observed. The subsequent lineage of each of these four cells is determined not by its ancestry (whether it was derived from B.al or B.ar), but rather by its newly assumed position (anterior or posterior). In other words, positional influences play a determining role in the developmental fates of these cells.
E cell, male (WA editor's note; new name= U) (figure 21 and figure 22): E.lp and E.rp are associated with the vas deferens where it joins the cloaca. E.rap and E.raa are in the pre-anal ganglion. Usually, E.la remains posterior to the preanal ganglion, but, in two individuals, it behaved like E.ra and migrated anteriorly and divided.
C cell, male (WA editor's note; new name= Y) (figure 21 and figure 22): The C cell generates compact, neuronal-like nuclei. C.a remains in the pre-anal ganglion, while C.p divides transversely and ultimately produces a pair of five-celled ganglia which flank the cloaca.
(b) Mesoderm
M cell, hermaphrodite (figure 6, figure 23, figure 24, and figure 25): All of the postembryonic mesodermal cell lineages derive from a single mesoblast, M. M divides dorso-ventrally about midway through the L1 stage (figure 15). Three subsequent divisions generate 16 daughter cells, 4 in each of the muscle quadrants. Two of the ventral cells (M.vlpa and M.vrpa) divide longitudinally once more, so that by the L1 molt 18 progeny cells are present. During the L2 stage, two of the dorsal cells (M.dlpa and M.drpa) differentiate into coelomocytes. It is interesting that all four of these "specialized" cells are lineally equivalent daughters from the four muscle quadrants. Two of the daughters of the "extra" ventral divisions (M.vlpaa and M.vrpaa) migrate anteriorly. The other 14 progeny cells (6 dorsal, 8 ventral) become normal body muscles and are used for locomotion. They intercalate with juvenile body muscles posterior to the gonad primordium. The precise pattern of this intercalation varies among different individuals. A typical arrangement is shown in figure 24. The mature adult thus contains 95 body muscle cells; the ventral right quadrant and each dorsal quadrant contains 24 of these cells, and the ventral left quadrant contains 23.
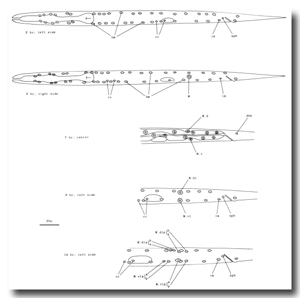 Figure 23. M; hermaphrodite, lateral views; mesodermal development during the L1 stage. bm, body muscle; cc, coelomocyte; dep, anal depressor muscle; im, intestinal muscle; sph, anal sphincter muscle.
Figure 23. M; hermaphrodite, lateral views; mesodermal development during the L1 stage. bm, body muscle; cc, coelomocyte; dep, anal depressor muscle; im, intestinal muscle; sph, anal sphincter muscle.
 Figure 24. M; hermaphrodite, left lateral views of the left side; mesodermal development. cc, coelomocyte.
Figure 24. M; hermaphrodite, left lateral views of the left side; mesodermal development. cc, coelomocyte.
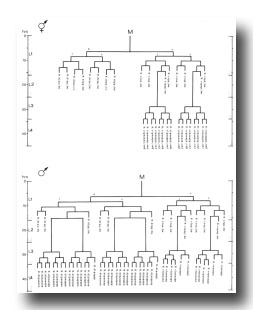 Figure 25. M lineage, hermaphrodite and male; mesodermal development. Divisions are anterior-posterior unless otherwise indicated. bm, body muscle; cc, coelomocyte; um1, type 1 uterine muscle; um2, type 2 uterine muscle; vml, type 1 vulval muscle; vm2, type 2 vulval muscle.
Figure 25. M lineage, hermaphrodite and male; mesodermal development. Divisions are anterior-posterior unless otherwise indicated. bm, body muscle; cc, coelomocyte; um1, type 1 uterine muscle; um2, type 2 uterine muscle; vml, type 1 vulval muscle; vm2, type 2 vulval muscle.
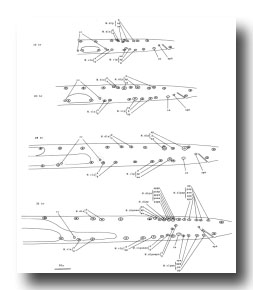 Figure 26. M; male tail, left lateral views of the left side; mesodermal development. cc, coelomocyte. The muscles continue to migrate in the L4.
Figure 26. M; male tail, left lateral views of the left side; mesodermal development. cc, coelomocyte. The muscles continue to migrate in the L4.
M.vlpaa and M.vrpaa continue their movement until late during the L2 stage, stopping when they reach a position midway along the developing gonad. Late during the L3 stage, these sex myoblasts begin a series of three longitudinal divisions. Each cell produces 2 sets of 4 daughters by the L3 molt, generating a total of 16 new cells located in 4 sectors around the dorso-ventral axis of the developing vulva. In each set, the cell nearest the vulva (e.g.,M.vlpaaapp) becomes a type 1 vulval muscle (vml); its sister.
(M.vlpaaapa) becomes a type 2 vulval muscle (vm2). vml attaches to the vulva more ventrally than does vm2 and attaches to the body wall more dorsally than vm2; vm1 is located more superficially than vm2. The cell farthest from the vulva in each set (e.g., M.vlpaaaaa) becomes a type 2 uterine muscle (um2); its sister (m.vlpaaaap) becomes a type 1 uterine muscle (um1). Both um1 and um2 form thin sheets with most of their filaments in a circumferential orientation. um1 attaches to the ventral side of the uterus close to the vulva and to the lateral ridge of the body wall. um2 attaches solely to the uterus in a region further from the vulva; in contrast to um1, um2 extends to the dorsal side of the uterus.
During the L4 stage, the nuclei of the sex muscles assume their final positions around the developing gonad. During the L4 molt, all of the 16 sex muscle cells twitch in characteristic directions. In the adult, these muscles are used in laying eggs.
M cell, male (figure 25 and figure 26): The mesodermal cell lineage in the male begins exactly as in the hermaphrodite. However, cells homologous with those which in the hermaphrodite differentiate into coelomocytes (M.dlpa and M.drpa) are blast cells in the male. The six male sex mesoblasts grow in size and, during the L3 stage, migrate posteriorly. Late during the L3 stage, these 6 cells begin a series of divisions, producing a total of 42 daughters. Most of the progeny of the ventral cells move to positions along the ventral side of (M.dlpa and M.drpa) are blast cells in the male. The six male sex mesoblasts grow in size and, during the L3 stage, migrate posteriorly. Late during the L3 stage, these 6 cells begin a series of divisions, producing a total of 42 daughters. Most of the progeny of the ventral cells move to positions along the ventral side of the nematode. Some of the daughters of the posterior dorsal cells migrate posteriorly into the post-anal region of the tail; some of the others move ventrally. Most of the daughters of the anterior dorsal cells migrate to the ventral side; most then continue their movements, some in an anterior direction and some in a posterior direction. These migrations, which have not yet been followed in detail, often occur concomitantly with the divisions which generate these cells; there is substantial variation among individuals regarding the precise order of events as well as the orientation of the division planes of the migrating cells. For example, sometimes a given cell will migrate from the dorsal to the ventral side and then divide, whereas sometimes this same cell will divide on the dorsal side and then both of its daughters will migrate to the ventral side.
We have not yet identified these 42 daughter cells in the adult male, but we believe they must include a variety of male-specific muscles located in the tail and, usually, one coelomocyte (located near the dorsal midline somewhat anterior to the anus). Approximately 16 of the muscles are located anterior to the anus and run diagonally in a ventral-posterior to lateral-anterior direction. Other male-specific muscles control the movements of the spicules.
(c) Intestine.
I cells (figure 14 and figure 16): Fifteen minutes after the beginning of L1 lethargus, the intestinal divisions take place. Normally, the six anterior-most nuclei do not divide; any of the four posterior-most nuclei may also fail to divide. Thus, division of 10-14 of the 20 juvenile intestinal nuclei produces the 30-34 nuclei normally found in the adult.
Other patterns of division occasionally occur. Once, for example, an intestinal nucleus located immediately behind the anterior six appeared to die during division; after anaphase, no daughter nuclei were formed. Concomitantly, the adjacent anterior nucleus divided, the only time this nucleus has been observed to do so.
(d) Gonad. The cell lineages of the gonad have not been determined. As suggested by Hirsh et al. (1976), the morphology of the gonad offers a convenient means of staging the postembryonic development of C. elegans. For comparison with the timing of other postembryonic events, various stages of gonad development in both the hermaphrodite and the male can be seen in figure 9, figure 24, and figure 26.
(4) Other Nematodes
Postembryonic developmental events similar to those we have observed in C. elegans also occur in other nematodes. We have studied the postembryonic development of a variety of nematode species in two ways: (1) observation of Feulgen-stained specimens to determine whether or not a postembryonic increase occurs in the number of cell bodies in the ventral cord, and (2) examination of living specimens using Nomarski optics to determine whether or not cell divisions could be observed directly. Based upon these criteria, postembryonic nongonadal cell divisions occur in all nematodes we have studied.
Panagrellus redivivus (the "sour-paste nematode") and Turbatrix aceti (the "vinegar eelworm") are free-living nematodes in a different family from that of C. elegans (de Coninck, 1965). Aphelencoides blastophthorus is plant parasitic and in a different order (de Coninck, 1965). All three of these species showed development of their ventral nerve cords and cell divisions in other tissues that appeared to be very similar to these events in C. elegans. A. blastophthorus may contain slightly more ventral cord neurons than C. elegans.
Ascaris lumbricoides var. suis, an intestinal parasite of pigs, is in an order different from both C. elegans and A. blastophthorus (de Coninck, 1965). It also showed development in its ventral cord similar to that seen in C. elegans. For microscopy, embryos and L1 larvae were artificially released from eggs at different developmental stages as described under Materials and Methods. (Normally, A. lumbricoides larvae do not hatch until the L2 stage.). The first cleavage of the egg occurred on Day 4; by Day 14, the embryos had reached the "comma" or "tadpole" stage (Chitwood and Chitwood, 1974). At Day 17, the eggs contained moving larvae with nuclei sparsely distributed in the ventral cord. By Day 21, the number of nuclei in the ventral cord had increased severalfold to a number comparable to that in C. elegans L2 larvae. Some specimens with intermediate cord counts contained cells in the ventral cord in the process of division. Because of difficulties in visibility, cell divisions in A. lumbricoides were not observed using Nomarski optics.
Longidorus macrosoma, a raspberry ectoparasite, is in a different subclass from the other nematode species examined (de Coninck, 1965). Its ventral cord was quite distinctive, containing about 140 neuron-like nuclei in the L1. As in the other nematodes, substantial development occurs after the L1 stage, increasing the number of ventral cord neurons to about 1000 by the beginning of the L3 stage. Cell division was not observed using Nomarski optics.
We have not traced lineages in any of these other nematodes.
Postembryonic increases in the number of cells in the ventral cord, hypodermis, and intestine of the free-living nematode Rhabditis anomala have been described by Wessing (1953). The increase in the number of ventral cord nuclei in P. redivivus has been previously reported by Hechler (1970).
 Figure 27. PLATE 8 - Darkly-staining junction of the somatic interneurone (I) with M1 and I1. Symbols as in table 1. (Magn. x 46350.)
Figure 27. PLATE 8 - Darkly-staining junction of the somatic interneurone (I) with M1 and I1. Symbols as in table 1. (Magn. x 46350.)
Discussion
(A) Summary
(B) Functions of Postembryonic Cell Divisions
(C) Invariance
(D) Patterns of Cell Divisions
(E) Mechanisms of Determination
(F) Symmetry
(G) Migrations
(F) Prospects
(A) Summary
The number of nongonadal nuclei in the hermaphrodite of C. elegans increases from about 550 at hatching to about 810 in the mature adult (Table 2). Essentially invariant cell lineages generate a fixed number of progeny cells of rigidly determined fates. These postembryonic cell lineages range in length from one to eight sequential divisions.
Table 2. Nuclear counts, hermaphrodite.
A number of these lineages produce the accessory sexual structures of the hermaphrodite: the vulva (through which eggs are laid), vulval and uterine muscles, and neurons which innervate these muscles. New neurons are also added to the motor nervous system. Almost one-third of all postembryonic progeny nuclei become part of the hypodermal syncytium of the nematode body wall. The number of intestinal nuclei almost doubles. There is a small increase in the number of body muscles. Few cell divisions occur in the head; both the anterior sensory nervous system and the pharynx appear to be complete at hatching.
In the development of the hypodermis, eight blast cells on each lateral side (H1, H2, V1-V6) follow similar programs of cell division to generate a total of 92 syncytial hypodermal nuclei and 28 seam cells. (Seam cells form a distinct cellular band embedded in the lateral hypodermal ridge.) These hypodermal cell divisions occur around the time of the first three larval molts. Two lateral tail blast cells (T) produce two seam and six syncytial nuclei. Twelve other syncytial hypodermal nuclei are produced in the ventral cord by precursors (P1-P4, P8-P12) primarily responsible for neuronal development. The other three ventral cord precursors (P5-P7) generate the 22-cell vulva, the hypodermal structure through which eggs are laid. In the young larva, the ventral cord precursors form an integral part of the hypodermal body wall.
Most of the neuronal development occurs in the ventral nerve cord and its associated ganglia. Identical patterns of division during the first larval stage of 12 precursor cells (P1-P12) and 1 neuroblast (P0.a) followed by a fixed program of cell deaths add 56 neurons to the ventral motor nervous system. Two of the lateral hypo-dermal blast cells (V5) produce small ganglia as well as the hypodermal nuclei mentioned above. Two other lateral hypodermal blast cells (T) and the lateral neuroblasts (Q1, Q2) also generate neurons and or glial cells.
Mesodermal development derives exclusively from a single cell (M) present in the newly hatched larva. A series of divisions during the first larval stage produces 14 body muscles, 2 coelomocytes, and 2 myoblasts. These myoblasts migrate anteriorly until they reach the position where the vulva will form. They divide shortly before the third molt to produce 16 sex muscles, which function in egg-laying.
Most of the intestinal nuclei (In) divide once, before the first molt.
A number of other cell divisions also occur in the hermaphrodite.
The postembryonic development of the male differs from that of the hermaphrodite. In the male, the number of nongonadal nuclei increases from about 550 to about 970 (Table 3). Most of the additional, male-specific cells are located in the specialized structures of the tail used by the male during copulation. Many cell lineages are similar in the male and hermaphrodite; the two sexes display virtually identical changes in the lateral hypodermis, body musculature, and intestine. Some male-specific cells are produced by lineages which are initially identical to those seen in the hermaphrodite. For example, extra divisions in the lateral hypodermal lineages (V5, V6, T) produce the 18 rays of the male tail. The mesodermal lineage (M) generates the male-specific sex muscles instead of the vulval and uterine muscles of the hermaphrodite. The development of the male ventral nerve cord involves extra cell divisions as well as fewer programmed cell deaths. Two posterior ventral cord hypodermal cells (P10.p, P11.p) divide to form part of the male tail, whereas the three cells homologous with those which produce the vulva in the hermaphrodite (P5.p, P6.p, P7.p) do not divide in the male. The other male-specific structures (the cloaca, spicules, and associated ganglia) are derived from cells (B, C, E, F) that are present in all L1 larvae but divide only in males.
Table 3. Nuclear counts, male.
The postembryonic development of C. elegans involves the sequential addition of new structures onto a preexisting larval edifice; no destruction of larval-specific cells occurs. However, there are structural modifications of larval cells: The "dorsal D" motoneurons of the ventral cord reverse their polarity during postembryonic development; in the L1, they receive innervation from the dorsal cord and synapse onto ventral body muscles, whereas in the adult they receive innervation from the ventral cord and synapse onto dorsal body muscles (J. G. White, personal communication).
(B) Functions of Postembryonic Cell Divisions
A number of the postembryonic cell lineages are related to sexual maturation. At hatching, the hermaphrodite and the male are almost identical with respect to cell number, position, and (presumably) function. Yet, by the adult stage, gross anatomical differences are obvious in nongonadal tissues. Most of the cell divisions which give rise to these sex-specific structures occur shortly before the third molt. In the hermaphrodite, ectodermal lineages produce the 22 cells which form the vulva, the structure through which eggs are laid. Mesodermal lineages generate the 16 muscle cells which control the vulva and uterus during egg-laying. Neurons which innervate at least some of these muscles (White et al., 1976) are also formed post-embryonically from the neuronal lineages of the ventral cord. In the male, related ectodermal, mesodermal, and neuronal lineages generate some of the structures of the tail which function during copulation. The other male-specific structures are produced by lineages derived from cells which in the hermaphrodite do not divide.
Some of the postembryonic cell divisions may relate to growth. For example, four rounds of hypodermal cell divisions during the first three larval stages substantially increase the number of hypodermal nuclei. The increases in the numbers of intestinal nuclei and body muscle cells may similarly reflect growth, although it is unclear why there are cell divisions in these tissues only during the first larval stage.
Explanations for other postembryonic cell divisions are not obvious. For example, the L1 moves quite adequately using its juvenile locomotory ventral nervous system; yet, the number of neurons in this system is almost tripled during early larval development. Perhaps for mechanical reasons the larger adult requires a more intricate nervous system to control its locomotion. Alternatively, the adult may be capable of behaviors more complex than those of the L1.
(C) Invariance
The postembryonic somatic cell lineages of C. elegans are generally invariant, with a fixed pattern of cell divisions and a fixed developmental program defined for every daughter cell. However, essentially five types of variations in the details of this developmental program have been observed, as follows. (1) Variation in the pattern of cell divisions: The greatest inconstancy appears in the single round of divisions of the intestinal nuclei; the four posterior most nuclei sometimes divide and sometimes do not. Slight variations occur in the patterns of divisions of the hypodermal cells of the ventral cord. Among males, P9.p occasionally divides; among hermaphrodites, P3.p sometimes fails to divide. Similarly, E.la occasionally divides in males. (2) Variation in the pattern of cell deaths: Q2.pp, which normally appears to die, has once been observed to survive into the adult. (3) Variation in which of two alternative lineage programs a given cell will follow: These cases appear to involve positional effects and are discussed below (see Mechanisms of Determination). (4) Variation in the precise order of specific events: For example, in mesodermal development, sometimes M.vla divides before M.vra, whereas sometimes M.vra divides before M.vla. Similar timing variations occur between lineages as well as within a given lineage. Migrations and divisions also are not rigidly ordered, because sometimes a cell will migrate and then divide, whereas sometimes it will divide and its daughters will migrate (see Results, Mesoderm). (5) Variation in the precise positions of cell nuclei: For example, the development of both the ventral nerve cord and the somatic musculature leads to variable intercalation of new progeny cells with the juvenile cells present at hatching. For this reason, our diagrams cannot be used to identify all cells in an animal which has already developed.
(D) Patterns of Cell Divisions
Two distinguishable types of cell divisions occur during the postembryonic development of C. elegans. Some divisions are symmetrical, producing daughters which are equivalent in morphology and, often, in subsequent development. Other divisions are asymmetrical, producing two distinctly different types of daughter cells; in at least some instances, asymmetric divisions generate a posterior daughter that is morphologically like its mother cell and an anterior daughter of a new cell type. Both types of divisions can be seen in the development of the lateral hypodermis (Fig. 10); for example, Vn.p divides symmetrically, whereas Vn.pa and Vn.pp divide asymmetrically. Asymmetric divisions almost always occur along an anterio-posterior axis and could reflect an anterio-posterior asymmetry of developmental determinants located either inside or outside the parent cell. That transverse divisions are symmetrical (see below, Symmetry) suggests that the distribution of such determinants may be bilaterally symmetric. The few dorsal-ventral divisions appear to be asymmetrical. However, the examples in the male specific B, C, and F lineages are difficult to interpret, because divisions in these lineages generally fail to conform to the orthogonal coordinate system seen elsewhere. The only other example (the first division of the mesoblast, M) produces progeny which follow identical lineages for three further divisions; the subsequent different fates of a few of the lineally equivalent dorsal and ventral cells (e.g., M.dlpa and M.vlpa) could arise from local environmental influences.
Comparison of the various cell lineages reveals that there is no standard type of cell lineage utilized for generating groups of new cells. Although most lineages involve both symmetrical and asymmetrical cell divisions, two exemplify extreme types of lineage logic. In the mesodermal lineage (Fig. 25), a series of symmetrical divisions adds a relatively large number of new cells of a particular type at one time. In the lateral hypodermal lineages (Fig. 10), a series of asymmetrical divisions adds sequentially individual progeny which differentiate into types distinct from the parent cell; in such stem cell lineages, one daughter always maintains the morphology of the original mother cell.
A variation of this "stem cell logic" is thought to produce the ventral nervous system of Drosophila melanogaster (Poulson, 1950; Seecoff et al., 1973) and may also be used by C. elegans. In Drosophila, each of a set of neuroblast stem cells generates another stem cell and a "predifferentiated" cell that divides once before differentiating. The lineage of the C. elegans ventral nervous system (particularly in the male; Fig. 17) is similar. Perhaps this type of lineage is common in neuronal development.
Another type of lineage that has been observed in insects may be utilized by C. elegans. In a number of insects, similar cell lineages generate scales, hairs, bristles, and sensilla (Lawrence, 1966); many of these insect lineages involve programmed cell death. In C. elegans, we find possible analogs in the development of the rays (probably mechanosensilla), the posterior lateral ganglia (associated with the postdeirid sensilla), and the lumbar ganglia (partially associated with the phasmid sensilla) (Fig. 27). Although there are difficulties in interpretation (see the legend to Fig. 27), we feel that the similarity of these lineages may reflect a fundamental subprogram of development.
 Figure 27. Comparison of lineages found in insects and C. elegans. Insects: Generalized from Lawrence (1966). The lineage tree does not reflect the division axes. Ray: The neuron assignment is based on the identification of dopamine in this cell. The assignment of the structural element is based upon experiments with the laser (see Results, Cell Lineages). Postdeirid: Assignments were made by comparing nuclear morphology and arrangement as seen with Nomarski optics with that observed in serial-section electron micrographs. Because individuals of known lineage have not been examined by electron microscopy, the assignments of the sheath and socket cells (defined by Ward et al., 1975) conceivably could be switched. Lumbar ganglion: assignments were made by tracing nuclei observed with Nomarski optics through to the adult stage and identifying these nuclei in serial-section electron micrographs. The wing-shaped accessory cell and the socket cell are associated with the phasmid. However, the three neurons apparently are not closely associated with this sensillum. Furthermore, the phasmid has another associated accessory cell which is present in the newly hatched larva. In this case, we would propose that the lineage defines the general functions of cells which subsequently assort into different sensory elements. The fates of some sister cells in the lumbar ganglion lineage are reversed compared to the fates of lineally equivalent cells in the ray and postdeirid lineages, possibly reflecting local environmental influences (see Mechanisms of Determination).
Figure 27. Comparison of lineages found in insects and C. elegans. Insects: Generalized from Lawrence (1966). The lineage tree does not reflect the division axes. Ray: The neuron assignment is based on the identification of dopamine in this cell. The assignment of the structural element is based upon experiments with the laser (see Results, Cell Lineages). Postdeirid: Assignments were made by comparing nuclear morphology and arrangement as seen with Nomarski optics with that observed in serial-section electron micrographs. Because individuals of known lineage have not been examined by electron microscopy, the assignments of the sheath and socket cells (defined by Ward et al., 1975) conceivably could be switched. Lumbar ganglion: assignments were made by tracing nuclei observed with Nomarski optics through to the adult stage and identifying these nuclei in serial-section electron micrographs. The wing-shaped accessory cell and the socket cell are associated with the phasmid. However, the three neurons apparently are not closely associated with this sensillum. Furthermore, the phasmid has another associated accessory cell which is present in the newly hatched larva. In this case, we would propose that the lineage defines the general functions of cells which subsequently assort into different sensory elements. The fates of some sister cells in the lumbar ganglion lineage are reversed compared to the fates of lineally equivalent cells in the ray and postdeirid lineages, possibly reflecting local environmental influences (see Mechanisms of Determination).
(E) Mechanisms of Determination
The strong correlation between lineage and function could arise in essentially two distinct (but not necessarily exclusive) ways. On the one hand, the ultimate differentiation of a cell could be determined extrinsically according to its position; alternatively, its fate could be determined intrinsically according to its lineage history. Our observations are consistent with the hypothesis that much of the development of C. elegans is based upon a lineal determination of cell function. The high degree of invariance of these lineages suggests such a mechanism. Furthermore, experiments with the laser microbeam system (see Results) suggest that the fates of specific cells in the posterior lateral ganglia, the ventral cord, and the rays of the male tail are autonomously determined; these fates remain invariant despite the fact that the undamaged cells sometimes differentiate in positions which normally would be occupied by cells with different fates. Preliminary experiments (J. White, J. Sulston, N. Thomson, E. Southgate, and R. Horvitz, unpublished results) utilizing mutants with abnormal cell lineages have also confirmed these observations; blocking one branch of the cell lineages of the posterior lateral ganglia or the ventral cord does not alter the developmental fates of cells generated by another branch.
There are, however, some developmental events in which position appears to have an influence. In the male tail in each of two cases (Bα and Bβ; Bγ and Bσ) — two cells of different lineage histories appear to be equally competent to follow either of two alternative developmental programs; their subsequent lineages are correlated with their relative positions and not with their previous histories. Similarly, ventral cord hypodermal blast cells P5.p and P6.p follow distinct programs in the development of the vulva, despite the fact that either of two cells present in the young L1 can become either P5 or P6; in other words, the developmental program that each of these cells follows is correlated with the position it assumes in the ventral cord. Two observations suggest that the gonad may be responsible for this positional influence on P5 and P6: (a) The lineages of the ventral hypodermal cells Pn.p are arranged essentially symmetrically around the midpoint of the developing gonad (Fig. 18), as if this point were acting as the source of a positional determinant, and (b) the Pn.p cells which divide are those immediately adjacent to the gonad. Other developmental events -e.g., the Pn.aap cell deaths in the ventral cord and the differentiation of the sex muscles — also occur symmetrically around the developing gonad of the hermaphrodite and similarly may reflect its influence.
Other aspects of the postembryonic development of C. elegans possibly may be affected by positional influences: (1) Those lineages which utilize sets of "equivalent" blast cells show apparent end effects; such effects include logical inversions (in which the fates of anterior and posterior daughters are reversed) and/or positional inversions (in which daughters migrate past one another), as well as modifications of the pattern of cell divisions itself. For example, such alterations are apparent in the H and T lineages (the head and tail equivalents of the V lineage; Fig. 10) and in the divisions, deaths, and ultimate cell fates at the ends of the ventral cord (Fig. 17 ; Table 1). These various modifications of cell lineages in the head and tail regions may reflect local environmental influences. (2) Six apparently equivalent developmental "regions" can be defined longitudinally along the lateral hypodermis of the nematode. In five of these regions, identical lineages are produced by the hypodermal blast cells. However, hypodermal blast cell V5 follows a modified developmental program to generate part of the posterior lateral ganglion. This region of the nematode is also unique in that it contains the lateral neuroblasts (Q) as well as the single mesoblast (M). Perhaps positional factors — possibly defined by one or more of these cells — determine the unique developmental aspects of this area of the nematode.
Although the examples described above indicate that cell lineages may be affected by positional influences, we do not know if the nematode is capable of regulation per se. In experiments involving the posterior lateral ganglia, the rays of the male tail, and the ventral cord, those structures normally derived from a cell which had been eliminated either by the laser or by mutation, were unequivocally absent in the adult; no regulatory processes induced extra divisions to compensate for the laser-or mutation-induced deficiencies. The unique observation of an abnormal death in the intestine followed by a compensatory abnormal division (see Results, Intestine) is a possible example of regulation.
(F) Symmetry
Although the young L1 is essentially bilaterally symmetrical, there are a variety of minor differences between its left and right sides which can be distinguished in the light microscope: (1) The gonad primordium lies obliquely, with its anterior portion displaced to the right and its posterior portion displaced to the left. In the region of the gonad primordium, the intestine is reciprocally displaced (anterior, left; posterior, right). (2) The mesoblast (M) is on the right. (3) The two coelomocytes on the right are anterior to those on the left. (4) There is an extra lateral hypodermal nucleus on the right side near the head and another on the left side in the tail. (5) There is an extra ventral body muscle nucleus on the right side posterior to the gonad primordium. (6) The lumbar ganglion on the right contains one more nucleus than that on the left. (7). The anal sphincter muscle nucleus is located on the left. (8) The excretory cell is displaced left of the midline.
Bilateral symmetry is reflected in the transverse divisions which occur during postembryonic development. Virtually all transverse divisions produce morphologically identical progeny which follow identical programs of development. The only indications of exceptions to this rule—(1) B.alapaav dies, B.arapaav does not; (2) Bγ.ald dies, Bγ.ard does not; (3) E.ra divides, E.la sometimes does not —may well arise from positional influences at the end of identical lineage programs.
The left and right lateral neuroblasts, Q1 and Q2, are bilaterally symmetrical in their initial positions and in their division programs. However, their progeny migrate differently. Q1 produces three progeny in the posterior half of the left side and Q2 produces three (or four) progeny in the anterior half of the right side.
The obliquely oriented gonad primordium of the young L1 shows twofold rotational symmetry around a dorso-ventral axis. The subsequent development of the hermaphrodite gonad, vulva, and sex muscles is also symmetrical about this dorso-ventral axis through the midpoint of the gonad.
(G) Migrations
Cellular migrations occur frequently during the postembryonic development of C. elegans. Because Nomarski optics does not allow clear visualization of cellular boundaries, we cannot always be certain that whole cells —as opposed to nuclei — are migrating. However, in most migrations, nucleus and cytoplasm do appear to move together. The migrations of the precursor (P) cells into the ventral cord are distinctive; a cytoplasmic extension moves into the cord and is followed by the nucleus and, finally, by the remaining cytoplasm. Some migrations — such as those in the posterior half of the ventral cord — appear to be passive events that depend solely on the activities of neighboring cells; often such movements are variable in extent from individual to individual. Other migrations are rigidly determined, with cells traversing relatively long distances from reproducible starting and stopping sites. The most striking of the migrations involve progeny of the lateral neuroblasts (Q1 and Q2) and the mesoblast (M): (1) Three of the progeny of Q2 (Q2.a, Q2.p, Q2.ap) travel rapidly along the left lateral hypodermis; they move about 50 micrometer (about 16 nuclear diameters) in 2 hr. Q1.ap shows a similar, but somewhat shorter, migration. (2) The two sex myoblasts of the hermaphrodite (M.vlpaa, M.vrpaa) migrate from their origin among the posterior ventral body muscles to the anterior-posterior center of the developing gonad; they move about 65 micrometer (about 16 nuclear diameters) in 6 hr. (3) Most of the daughters of the dorsal anterior sex mesoblasts of the male (M.dlpaa, M.drpaa) migrate from the region of the dorsal body muscles around to that of the ventral body muscles; they move about 20 micrometer (about 5 nuclear diameters) in 1 hr. The movements of these male-specific mesoblasts occur independently of the concomitant divisions of these cells. As described above (see Results, Mesoderm), sometimes a given cell will migrate and then divide; sometimes it will divide and then both daughters will migrate; and sometimes it will start to migrate, divide, and then the two daughters will complete the migration.
(H) Prospects
The postembryonic development of C. elegans offers a variety of opportunities for further exploration: (1) This development is rigidly determined; specific cells follow precise developmental programs at well-defined times. This system can now be used to obtain a detailed analysis at the fine-structure level of such developmental events as cell migration and synapse formation as well as other types of cellular differentiation. For example, the morphological changes associated with programmed cell death could be studied for a specific cell; the sequence of events could be traced by examining individuals at different stages from the time that cell is born to the time it disappears. (2) The laser system developed by J. G. White will allow exploration of possible intercellular communications during the processes of cell divisions, migration, death, and differentiation. In addition, this laser system can be used to determine additional cell assignments [as described above (see Results) for the posterior lateral ganglia and the rays in the male tail] and cell functions. (3) Mutants which affect the cell lineages of postembryonic development may similarly be used to examine regulative effects and to determine cell assignments and functions. Furthermore, such mutants might provide clues concerning the logical bases of the genetic program for the postembryonic development of C. elegans.
Acknowledgments
We are indebted to Donna Albertson, Lois Edgar, Nichol Thomson, and John White for providing electron micrographs with which we could compare our observations from the light microscope. We thank David Hooper for providing us with various nematode strains, Marilyn Dew for assistance, John White for use of the laser microbeam system, and Sydney Brenner for stimulating our interest in C. elegans. We also thank our many colleagues for much detailed background information and stimulating discussions. H.R.H. was supported by a research fellowship from the Muscular Dystrophy Associations of America.
References
ALBERTSON, D. G., and THOMSON, J. N. (1976). The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B. 275, 299-325.
BERNS, M. W. (1972). Partial cell irradiation with a tunable organic dye laser. Nature (London) 240, 483-485.
BIRD, A. F. (1971). "The Structure of Nematodes." Academic Press, New York.
BORNSTEIN, M. B. (1958). Reconstituted rat-tail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab. Invest. 1, 134-137.
BRENNER, S. (1973). The genetics of behaviour. Brit. Med. Bull. 29, 269-271.
BRENNER, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
CASSADA, R. C., and RUSSELL, R. L. (1975). The dauerlarva, a postembryonic developmental variant of the nematode Caenorhabditis elegans. Develop. Biol. 46, 326-342.
CHITWOOD, B. G., and CHITWOOD, M. B. (1974). "Introduction to Nematology." University Park Press, Baltimore.
DE CONINCK, L. (1965). Systematique des nematodes. In "Traite de Zoologie" (P.-P. Grasse, ed.), Vol. 4 (Parts 2 and 3), pp. 586-1200. Masson et Cie, Paris.
HECHLER, H. C. (1970). Reproduction, chromosome number, and postembryonic development of Pana-grellus redivivus (Nematoda:Cephalobidae). J. Nematol. 2, 355-361.
HIRSH, D., OPPENHEIM, D., and KLASS, M. (1976). Development of the reproductive system of Caenorhabditis elegans. Develop. Biol. 49, 200-219.
HODGKIN, J. A. (1974). "Genetic and Anatomical Aspects of the Caenorhabditis elegans Male." Ph.D. thesis, University of Cambridge, Cambridge, England.
LAWRENCE, P. A. (1966). Development and determination of hairs and bristles in the milkweed bug, Oncopeltus fasciatus (Lygacidae, Hemiptera). J. Cell Sci. 1, 475-498.
PEARSE, A. G. E. (1968). "Histochemistry, Theoretical and Applied," Vol. 1, p. 603. Churchill, London.
POULSON, D. F. (1950). Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster Meigen. In "Biology of Drosophila" (M. Demerec, ed.), pp. 168-274. Hafner, New York.
RAFALKO, J. S. (1946). A modified Feulgen technic for small and diffuse chromatin elements. Stain Technol. 21, 91-93.
ROGERS, W. P. (1960). The physiology of infective processes of nematode parasites: The stimulus from the animal host. Proc. Roy. Soc. Lond. B. 152, 367-386.
SAUNDERS, J. W. (1966). Death in embryonic systems. Science 154, 604-612.
SEECOF, R. L., DONADY, J. J., and TEPLITZ, R. L. (1973). Differentiation of Drosophila neuroblasts to form ganglion-like clusters of neurons in vitro. Cell Diff. 2, 143-149.
SHIELDS, G. DUBENDORFER, A., and SANG, J. H. (1975). Differentiation in vitro of larval cell types from early embryonic cells of Drosophila melanogaster. J. Embryol. Exp. Morphol. 33, 159-175.
SULSTON, J. E. (1976). Post-embryonic development in the ventral cord of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B. 275, 287-297.
SULSTON, J., and BRENNER, S. (1974). The DNA of Caenorhabditis elegans. Genetics 77, 95-104.
SULSTON, J., DEW, M., and BRENNER, S. (1975). Do-paminergic neurons in the nematode Caenorhabditis elegans. J. Comp. Nenrol. 163, 215-226.
WARD, S., THOMSON, N., WHITE, J. G., and BRENNER, S. (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160, 313-338.
WARE, R. W., CLARK, C., CROSSLAND, K., and RUSSELL, R. L. (1975). The nerve ring of the nematode Caenorhabditis elegans. J. Comp. Neurol. 162, 71-110.
WEINSTEIN, P. P. (1952). Regulation of water balance as a function of the excretory system of the filariform larvae of Nippostrongylus muris and Ancyclostoma caninum. Exp. Parasitol. 1, 363-376.
WESSING, A. (1953). Histologische Studien zu den Problemen der Zellkonstanz: Untersuchungen an Rhabditis anomala P. Hertwig. Zool. Jb. 73, 69-102.
WHITE, J. G. (1974). "Computer-Aided Reconstruction of the Nervous System of C. elegans." Ph.D. thesis, University of Cambridge, Cambridge, England.
WHITE, J. G., SOUTHGATE, E., THOMSON, J. N., and BRENNER, S. (1976). The structure of the ventral nerve cord of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B. 275, 327-348.
WILSON, E. B. (1925). "The Cell in Heredity and Development." MacMillan, New York.
|