|
Postembryonic Cell Lineages of the Hermaphrodite and Male Gonads in
Caenorhabditis elegans
Judith Kimble and David Hirsh
Department of Molecular, Cellular and Developmental Biology
University of Colorado, Boulder, Colorado 80309
Developmental Biology (1979) 70: 396-417
doi: 10.1016/0012-1606(79)90035-6
(Received August 24, 1978; accepted January 8, 1979)
Abstract - Introduction -
Material & Methods -
Results -
Discussion -
Acknowledgments
- References
Abstract
cells (Z1 and Z4) that are present in the newly hatched larvae of both sexes. The lineages of Z1 and Z4 are essentially invariant. In hermaphrodites, they give rise to a symmetrical group of structures consisting of 143 cells, and in males, they give rise to an asymmetrical group of structures consisting of 56 cells. The male gonad can be distinguished from the hermaphrodite gonad soon after the first division of Z1 and Z4. However, the development of Zl and Z4 in hermaphrodites shares several features in common with their development in males suggesting that the two programs are controlled by similar mechanisms. In the hermaphrodite lineage, a variability in the positions of two cells is correlated with a variability in the lineages of four cells. This variability suggests that cell-cell interaction may play a more significant role in organisms that develop by invariant lineages than has hitherto been considered. None of the somatic structures (e.g., uterus, spermatheca, vas deferens) develops as a clone of a single cell. Instead, cells that arise early in the Z1-Z4 lineage generally contribute descendants to more than one structure, and individual structures consist of descendants of more than one lineage.
Introduction
How the developmental history of a cell influences its capacity to differentiate persists as one of the central questions in developmental biology. Cell lineages pose this fundamental question in a framework that permits an analysis of specific developmental events. Classically, cell lineage studies were used to explore the organization of the zygote and the basis of cell determination during embryogenesis. From these studies came the idea that many eggs are mosaics of cytoplasmic determinants that are distributed to specific cells by an invariant pattern of cell divisions (see Wilson, 1925).
The nematode Caenorhabditis elegans is an excellent organism for lineage studies because it has a limited number of cells and is genetically well defined (Brenner, 1974). Since C. elegans is transparent, individual cells can be distinguished microscopically and followed in living worms. The genetic control of lineage patterns can be studied using mutations that alter known lineages. Such mutations also provide a means of perturbing the normal pattern to investigate how changes in cell divisions or positions affect subsequent development. A similar method of approach to the genetic control of cell lineages was used by Lees and Waddington (1942) using the bristle lineage in Drosophila as a model system. However, they were required to infer developmental events from fixed specimens.
Considerable work has already been done on lineages in C. elegans. A description of the Postembryonic nongonadal lineages has been completed (Sulston and Horvitz, 1977), and work on the embryonic lineages is in progress (Deppe et al., 1978). Laser ablation experiments have demonstrated that cells are rigidly determined in at least some lineages (Sulston and Horvitz, 1977). Analysis of a lineage mutant in C. elegans has shown that nerve precursor cells blocked in division are still capable of exhibiting the differentiated phenotypes of their descendants (Albertson et al., 1978).
Previous studies in our laboratory have included a morphological description of the developing gonads in hermaphrodites and males (Hirsh et al, 1976; Klass et al, 1976), and the isolation of temperature-sensitive gonadogenesis-defective mutants (Hirsh and Vanderslice, 1976). This paper presents the postembryonic gonadal lineages of C. elegans. The lineages of the germ line progenitor cells are variable from worm to worm, and are discussed only briefly here. Although clonal information may be relevant to the generation of oocytes and sperm, other methods, such as laser ablation of precursor cells, will be necessary to elucidate the germ line lineages. By contrast, the lineages which generate the somatic structures of the gonad proceed according to an essentially invariant pattern.
The lineages that give rise to the somatic structures of the gonad are of particular interest because they display a complete program of development from only two cells, which are present when the worm hatches, to multiple cell types that are organized into discrete organs in the adult gonad. Although the embryonic ancestry of the two somatic progenitor cells is not known in C. elegans, Pai (1927) reported that the equivalent two cells of another nematode,Turbatrix aceti, arose from an embryonic stem cell, S5, as sisters. Since work on the embryonic lineage of C. elegans is in progress (Deppe et al., 1978), the complete lineage of these structures should be known. Another interesting aspect of this lineage is that the two cells follow one developmental pattern in the hermaphrodite and another in the male, so that a comparison of homologous programs is possible.
Materials & Methods
Nematode strain. Wild-type C. elegans var. Bristol, designated N2, is from the University of Colorado, Boulder, stock. The nematodes were handled as described by Brenner (1974) and Hirsh et al. (1976). Worms for lineage studies were kept at either 16 or 20°C until they were picked for observation.
Technique to obtain cell lineages. We used the technique of Sulston and Horvitz (1977) for mounting live worms for observation over long periods of time. Briefly, a worm is placed on a thin agar pad on a microscope slide. A slurry of E. coli is applied in a small disk to a coverslip. The coverslip is placed so that the worm is trapped between the agar surface and the coverslip in a thin layer of S medium (0.1 M NaCl and 0.05 M KH2PO4, pH 6.0). The worm is able to crawl on the agar surface and feed on the bacteria supplied. The edge of the coverslip is sealed with Vaseline or immersion oil so that the preparation does not dry out.
Our observations were made using a Zeiss Universal microscope equipped with Plan 100 objective and Nomarski differential interference contrast optics. One limit of this technique is that cell boundaries are indistinct. Thus, the bulk of the lineage data reflects the behavior of cell nuclei. Drawings were made as frequently as possible during periods of active migration and rapid division, and as frequently as necessary (every 0.5 to 2 hr) when nuclei were less active. The complete lineages were compiled from observations of over 50 hermaphrodites and 20 males. Photomicrographs were taken with a Zeiss microflash illuminator.
Nomenclature. We have used the nomenclature of Sulston and Horvitz (1977) for naming cells. A cell's daughters are each named by adding to the name of the mother cell a single lower case letter describing the position of each daughter relative to the other just after division (a = anterior, p = posterior, d = dorsal, v = ventral, 1 = left, r = right). Thus, if the cleavage plane of Z1 is oriented perpendicular to the anterior-posterior axis, the resulting anterior daughter is identified as Z1.a and the posterior daughter as Z1.p. If a division occurs obliquely, the resulting daughters are identified by adding a single letter signifying one axis only.
Lineage charts. The lineage trees presented here follow the rules formulated by Sulston and Horvitz (1977) as far as possible. A division is depicted by a branch point in which the left branch represents an anterior, dorsal, or left daughter and the right branch represents a posterior, ventral, or right daughter. Branches are marked by the same letters used in naming cells except that combinations of letters are used to describe oblique divisions. In the case of simple anterior-posterior divisions, the letters are omitted. In each lineage chart, the vertical coordinate represents time. Since the lineages are obtained at varying temperatures (19-23°C), the times divisions take place are normalized to 20°C. The times at which individual molts occur have been used as time standards for this correction. The horizontal coordinate of the lineage chart represents the anterior-posterior axis with anterior to the left for the entire hermaphrodite lineage and the first half of the male lineage. In the male lineage chart, the horizontal coordinate system in the last half of the divisions represents the distal-proximal axis with proximal to the left. The proximal end of the gonad is the opening at the cloaca; the distal is the farthest from the opening. Anterior, then, is to the left in the chart until the gonad reflexes, and it is to the right for the rest of the divisions. This is necessary, because in males, unlike hermaphrodites, divisions occur in the bend of the gonad. Since these divisions take place at variable points in the bend, the positions of the daughters vary with respect to the coordinates of the worm. However, the divisions are invariant when viewed with respect to the distal-proximal axis of the gonad.
Some variability is observed in the times at which divisions occur and in the orientation of cleavage planes. For example, in some worms Z1 divides first, in others Z4 divides first and, in a rare worm, they divide at the same time. Since they almost always divide within an hour of each other, they are represented in the lineage chart as dividing at the same time. A similar limited variability is observed in the cleavage planes, and here too, an average is shown in the lineage charts.
One-micron sections. Adult hermaphrodites were cut posterior to the pharynx or anterior to the anus in a drop of 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). A good cut will release the anterior or posterior gonadal arm intact into the fixative, though it remains attached to the worm by the somatic structures. The dissected animals were fixed in 2% glutaraldehyde for 1 hr, postfixed in 1% OsO4 for 1 hr, stained en bloc in 1% uranyl acetate for 1 hr, dehydrated in increasing concentrations of ethanol, transferred to propylene oxide, and embedded in Epon. Serial sections of 1micrometer thickness stained with toluidine blue were examined by light microscopy.
Results
Hirsh et al. (1976) and Klass et al. (1976) have described the major features of post-embryonic gonadogenesis in hermaphrodites and males, respectively. Figure 1 summarizes these findings. In both sexes, gonadal development starts from a four-celled gonadal primordium present at hatching and proceeds through all four larval stages (L1, L2, L3, and L4). Hermaphrodite gonadogenesis is characterized by twofold rotational symmetry, whereas male gonadogenesis is asymmetrical. Both hermaphrodite and male gonads display a distal-proximal axis with proximal toward the vulva in hermaphrodites and toward the cloaca in the male. The maturation of gametes occurs from distal to proximal. The somatic structures are located most proximally in the reproductive system.
 Figure 1. Overview of gonad development in hermaphrodites and males. At the top, the four celled gonadal primordium is shown in its midventral position in the newly hatched worm. In hermaphrodites (left column), the developing gonad elongates anteriorly and posteriorly during L1, L2, and L3. The growing tips reflex around the time of the L3-L4 molt. In males (right column), the developing gonad initially grows only in an anterior direction, and reflexes at about the L2-L3 molt. The main somatic structures of the adult hermaphrodite and male are shown schematically in the bottom two drawings, dtc, distal tip cell; ac, anchor cell; lc, linker cell; a. sp., anterior spermatheca; p. sp., posterior spermatheca.
Figure 1. Overview of gonad development in hermaphrodites and males. At the top, the four celled gonadal primordium is shown in its midventral position in the newly hatched worm. In hermaphrodites (left column), the developing gonad elongates anteriorly and posteriorly during L1, L2, and L3. The growing tips reflex around the time of the L3-L4 molt. In males (right column), the developing gonad initially grows only in an anterior direction, and reflexes at about the L2-L3 molt. The main somatic structures of the adult hermaphrodite and male are shown schematically in the bottom two drawings, dtc, distal tip cell; ac, anchor cell; lc, linker cell; a. sp., anterior spermatheca; p. sp., posterior spermatheca.
The gonadal primordium in the hermaphrodite is indistinguishable morphologically from that of the male. In each sex, the four primordial cells are located mid-ventrally along the anterior-posterior axis (figure 2A). These four cells are named Z1, Z2, Z3, and Z4 from anterior to posterior. Z1 and Z2 are located to the right of the worm's midsagittal plane, and Z3 and Z4 are to the left of that plane (figure 2B). Thus, the primordium exhibits twofold rotational symmetry. Although the hermaphrodite and male primordia are equivalent structures, newly hatched worms can be easily sexed according to three secondary sex characteristics recognized by Sulston and Horvitz (1977).
The organization of the somatic and germ line progenitor cells in the four-celled gonadal primordium of nematodes was revealed many years ago (reviewed in Chitwood and Chitwood, 1950). In both hermaphrodite and male worms, cells Z1 and Z4 give rise to the somatic structures of the gonad, and Z2 and Z3 give rise to the germ line cells. The primary focus of this paper is the Z1-Z4 somatic lineage. The cell lineages of Z2 and Z3 are variable in both sexes with respect to the planes of cell division and the times at which particular divisions occur. Z2-Z3 divisions occur continuously from L1 through adulthood. In hermaphrodites, the anterior and posterior gonadal arms each contain descendants of both Z2 and Z3. The plane of cell division can be left-right, dorsal-ventral, anterior-posterior, or oblique along any axis. A particular cell can divide relatively soon after its birth or it can be considerably delayed. No migrations have been observed among the germ line cells.
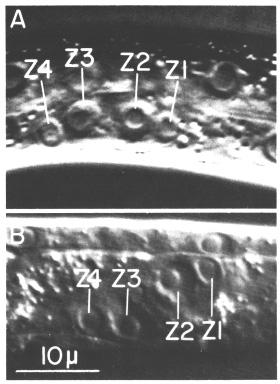 Figure 2. Gonadal primordium in young L1 hermaphrodite; Nomarski optics. A. Lateral view. This worm is unusual in that Z1, Z2, Z3, and Z4 are all visible in the same focal plane. B. Ventral view.
Figure 2. Gonadal primordium in young L1 hermaphrodite; Nomarski optics. A. Lateral view. This worm is unusual in that Z1, Z2, Z3, and Z4 are all visible in the same focal plane. B. Ventral view.
In contrast to the germ line cells, the somatic progenitor cells, Z1 and Z4, follow a nearly invariant pattern of cell divisions, migrations, and differentiation. The timing of events and the orientation of cleavages are essentially the same from worm to worm. The lineages of Z1 and Z4 are characterized by two periods of mitoses. The earlier period occurs during the first larval stage (L1) and the later one spans L3 and extends into L4.
Hermaphrodite Z1-Z4 Lineage (See figure 3)
Early Mitotic Period
Z1 and Z4 start dividing midway during the first larval stage (L1), and give rise to 12 cells by the L1-L2 molt, or shortly thereafter. After the first division, the anterior daughter of Z1, Z1.a, and the posterior daughter of Z4, Z4.p, occupy respectively the anterior and posterior tips of the primordium, and Z1.p and Z4.a lie ventral to the germ line cells (figure 4a). When Z1.a and Z4.p divide, Z1.aa and Z4.pp remain at the anterior and posterior tips of the gonad, and their siblings, Z1.ap and Z4.pa become positioned more dorsally in the primordium (figure 4b). Z1.p and Z4.a divide asymmetrically, and each gives rise to one larger daughter (Z1.pa and Z4.ap) and one small daughter (Z1.pp and Z4.aa) (figure 4b). Z1.pa, Z1.pp, Z4.aa, and Z4.ap, lying ventrally in the primordium, each divide to complete the early divisions (figure 4c). By the time of this final round of divisions, Z1.pa has moved to the left side, and Z4.ap has moved to the right side of the developing gonad. The daughters arising from these cells remain on the side to which the mother cell moved previously (figure 4d). Thus, four descendants of Z1 now lie on the opposite side that Z1 originally occupied and similarly for the descendants of Z4 (cf. figure 2 and figure 4d). Thus, the division pattern and cell movements carried out by Z1 are related by twofold rotational symmetry to the division pattern and cell movements of Z4.
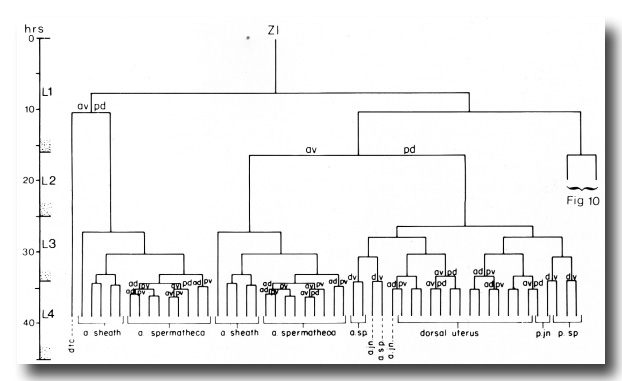 Figure 3. Hermaphrodite lineage of gonadal somatic progenitor cells Z1 and Z4. Only the invariant lineages are shown here; the variant lineages are shown in figure 10. Divisions give rise to an anterior daughter and a posterior daughter unless otherwise indicated. Anterior is to the left. The fates of descendants are indicated either individually or in groups; a, anterior; p, posterior; dtc, distal tip cell; sp, spermatheca; jn, spermathecal-uterine junction.
Figure 3. Hermaphrodite lineage of gonadal somatic progenitor cells Z1 and Z4. Only the invariant lineages are shown here; the variant lineages are shown in figure 10. Divisions give rise to an anterior daughter and a posterior daughter unless otherwise indicated. Anterior is to the left. The fates of descendants are indicated either individually or in groups; a, anterior; p, posterior; dtc, distal tip cell; sp, spermatheca; jn, spermathecal-uterine junction.
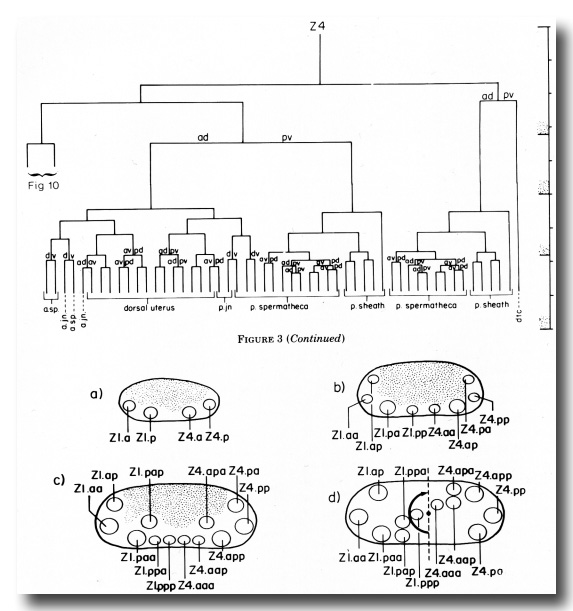 Figure 4. Spatial arrangement of hermaphrodite Z1 and Z4 descendant nuclei during the early mitotic period. The space occupied by Z2 and Z3 descendants is depicted by stippling, (a), (b), and (c) give lateral views after each round of divisions; (d) is a dorsal view of the same stage as (c) and shows that the positions of Z1 descendants are related to the positions of Z4 descendants by twofold rotational symmetry. The axis of symmetry is depicted as a dot, and the rotational operation is indicated by an arrow.
Figure 4. Spatial arrangement of hermaphrodite Z1 and Z4 descendant nuclei during the early mitotic period. The space occupied by Z2 and Z3 descendants is depicted by stippling, (a), (b), and (c) give lateral views after each round of divisions; (d) is a dorsal view of the same stage as (c) and shows that the positions of Z1 descendants are related to the positions of Z4 descendants by twofold rotational symmetry. The axis of symmetry is depicted as a dot, and the rotational operation is indicated by an arrow.
Amitotic Period
The somatic cells of the gonad do not divide again until late L2 or early L3, but the germ line cells approximately quadruple in number during L2. Thus, the somatic cells become separated from each other by an increasing number of germ line cells. Z1.aa remains at the anterior tip and Z4.pp at the posterior tip. Since the growing tips are the future distal tips in hermaphrodites, Z1.aa and Z4.pp are called distal tip cells. These two cells appear to serve a leader function, preceding the extending gonadal arms throughout gonadogenesis (figure 5). They do not divide again and they remain at the gonadal tips even in the adult. During L2, the gonadal primordium elongates from 25-30 to 50-70 micrometer.
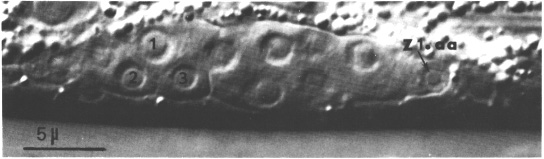 Figure 5. Hermaphrodite developing gonad after formation of the somatic primordium; Nomarski optics. This focal plane shows the anterior arm (Z2 and Z3 descendants), the anterior distal tip cell (Z1.aa), and three cells in the somatic primordium: 1, Z4.apa; 2, Z4.aap; 3, Z4.aaa. ganglion is indicated.
Figure 5. Hermaphrodite developing gonad after formation of the somatic primordium; Nomarski optics. This focal plane shows the anterior arm (Z2 and Z3 descendants), the anterior distal tip cell (Z1.aa), and three cells in the somatic primordium: 1, Z4.apa; 2, Z4.aap; 3, Z4.aaa. ganglion is indicated.
The second half of L2 is characterized by a marked growth without cell division and a positional change of ten Z1 and Z4 descendants (Z1.aa and Z4.pp remain at the distal tips). Just before the L2-L3 molt, these ten cells, five Z1 descendants and five Z4 descendants, move to the center of the gonad and overlap each other. During this process, the germ line cells are completely displaced from the central region. This rearrangement of somatic cells forms the hermaphrodite somatic primordium (figure 5). This primordium represents a new association of all the cells that will divide further to generate the individual somatic structures of the hermaphrodite gonad.
The ten cells arrange themselves in one of two alternative configurations in the somatic primordium (figure 6). The positions of eight of the somatic primordial cells are invariant. However, two cells, Z1.ppp and Z4.aaa, assume one of two alternative positions. Late in L2, either Z1.ppp or Z4.aaa moves into the midsagittal plane on the ventral surface of the gonad. Whichever cell acquires this midsagittal position is fated to become the anchor cell-a small, round cell which divides no further, and seems to be involved in the formation of the vulva. If Z1.ppp moves in from the left side of the gonad, only four cells remain on the left, whereas five cells are present on the right. This is called the 5R configuration (figure 6a). Conversely, if Z4.aaa moves in from the right side, four remain on the right and five on the left. This is the 5L configuration (figure 6b). Individual worms that developed by passing through a 5R configuration were observed to give rise to 5L and 5R progeny. Similarly, progeny of 5L worms can develop through either the 5L or 5R pathway.
 Figure 6. 5R and 5L somatic primordia, dorsal view. The two alternate cell arrangements are related to each other by a 180° rotation around the dorsal-ventral axis passing through the anchor cell (ac). Anterior is to the left.
Figure 6. 5R and 5L somatic primordia, dorsal view. The two alternate cell arrangements are related to each other by a 180° rotation around the dorsal-ventral axis passing through the anchor cell (ac). Anterior is to the left.
Late Mitotic Period
During L3 and L4, nine somatic cells (all the cells in the somatic primordium except the anchor cell) divide to generate 140 cells for a total of 143 cells in the L4, including the distal tip cells and the anchor cell. These cell divisions and subsequent differentiation give rise to five somatic structures: the anterior and posterior sheaths which encapsulate the germ line component of the gonad, the anterior and posterior spermathecae which store sperm from each gonadal arm, and a central uterus (figure 7).
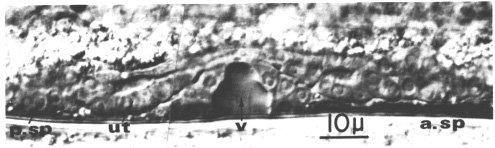 Figure 7. Hermaphrodite somatic structures at the mid-L4 stage; Nomarski optics. a. sp., anterior spermatheca; p. sp., posterior spermatheca, ut., uterus; v., vulval lumen.
Figure 7. Hermaphrodite somatic structures at the mid-L4 stage; Nomarski optics. a. sp., anterior spermatheca; p. sp., posterior spermatheca, ut., uterus; v., vulval lumen.
a) Sheath lineage. Two cells (Z1.ap and Z1.paa) in the somatic primordium each contribute five descendants to the anterior sheath, and two (Z4.pa and Z4.app) contribute five descendants to the posterior sheath (figure 8). Thus, each sheath consists of 10 cells. The sheath precursor cells also give rise to a major portion of the spermatheca.
The future sheath cells arise next to the developing spermatheca. They become flat elongated cells and spread over the surface of the germ line tube. They either migrate distally or are dragged along as the germ line arm elongates. Unless the nuclei stay along the visible edge of the gonadal arm, the sheath cells are very difficult to see. Therefore, 1-µm serial sections were cut from dissected gonads embedded in Epon, and stained with toluidine blue. The sheath cell nuclei, easily visible in cross section, were counted to make sure that no further division had occurred, and to ascertain the adult positions of these cells (figure 8).
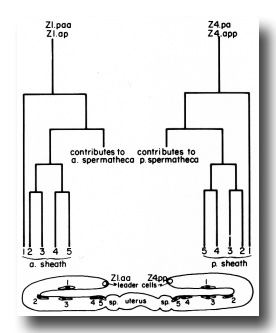 Figure 8. Correlation of lineage with adult position of the hermaphrodite sheath cell nuclei. Z1.paa and Z1.ap both follow the indicated lineage pattern to give rise to the anterior sheath. Z4.pa and Z4.app give rise to the posterior sheath. Thus, two descendants, one from each precursor cell, are found at each position (1, 2, 3, 4, and 5). After a sheath cell arises, it becomes flat and begins to migrate distally onto the germ line arm. The positions marked in the diagram were ascertained by a combination of direct observation of this migration and tracing the positions of cells in serial 1-µm sections of gonads embedded in Epon. The identity of the cells in positions 4 and 5 is known by direct observation. The identity of cells in position 1 is assigned because the distal daughters of the sheath precursor cells arise early in the lineage and their positions are already quite distal along the germ line arm when the other sheath cells arise. Identity of cells in positions 2 and 3 simply follows the positions of these cells for the first few hours after they arise. An exchange in their positions at a later time is possible, but has not been observed.
Figure 8. Correlation of lineage with adult position of the hermaphrodite sheath cell nuclei. Z1.paa and Z1.ap both follow the indicated lineage pattern to give rise to the anterior sheath. Z4.pa and Z4.app give rise to the posterior sheath. Thus, two descendants, one from each precursor cell, are found at each position (1, 2, 3, 4, and 5). After a sheath cell arises, it becomes flat and begins to migrate distally onto the germ line arm. The positions marked in the diagram were ascertained by a combination of direct observation of this migration and tracing the positions of cells in serial 1-µm sections of gonads embedded in Epon. The identity of the cells in positions 4 and 5 is known by direct observation. The identity of cells in position 1 is assigned because the distal daughters of the sheath precursor cells arise early in the lineage and their positions are already quite distal along the germ line arm when the other sheath cells arise. Identity of cells in positions 2 and 3 simply follows the positions of these cells for the first few hours after they arise. An exchange in their positions at a later time is possible, but has not been observed.
(b) Spermathecal lineage. The anterior and posterior spermathecae each arise from four cells of the somatic primordium (figure 9). Two cells each contribute nine descendants, and two cells each contribute three descendants.
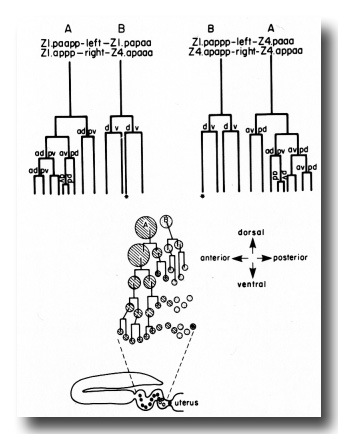 Figure 9. Schematic of spermatheca development. Four cells contribute descendants to each spermatheca. Two cells (A) each contribute nine, and two cells (B) each contribute three. The formation of only half of the spermatheca (either the right half or the left half) is depicted diagrammatically. The shaded circles represent A descendants, and the stippled circles represent B descendants. The starred cells form the core of the spermathecal-uterine junction.
Figure 9. Schematic of spermatheca development. Four cells contribute descendants to each spermatheca. Two cells (A) each contribute nine, and two cells (B) each contribute three. The formation of only half of the spermatheca (either the right half or the left half) is depicted diagrammatically. The shaded circles represent A descendants, and the stippled circles represent B descendants. The starred cells form the core of the spermathecal-uterine junction.
The spermathecae develop as spiral structures that join the developing uterus to the anterior or posterior sheath. Each spermatheca has a single left-handed twist in it along its longitudinal axis. These two structures are related by twofold rotational symmetry. As the spermathecal cells differentiate, the nuclei become small and irregular. The cytoplasm takes on a granular appearance and it becomes impossible to see the cytoplasmic boundaries. A lumen forms as the last divisions occur in the developing spermatheca. The adult spermatheca is a flexible tube that is continually contorted by the muscular contractions of the proximal arm sheath and the uterus.
(c) Uterine lineage. The dorsal portion of the uterus arises invariantly from two cells in the somatic primordium (figure 3). The ventral uterus can arise according to either of two alternative lineages (figure 10). These two lineages correspond to the two alternative configurations of the somatic primordium. One lineage is followed if the cells assume the 5R configuration (figure 10a), whereas another is followed from the 5L configuration (figure 10b). These two lineages, expressed in different animals, are related by twofold rotational symmetry.
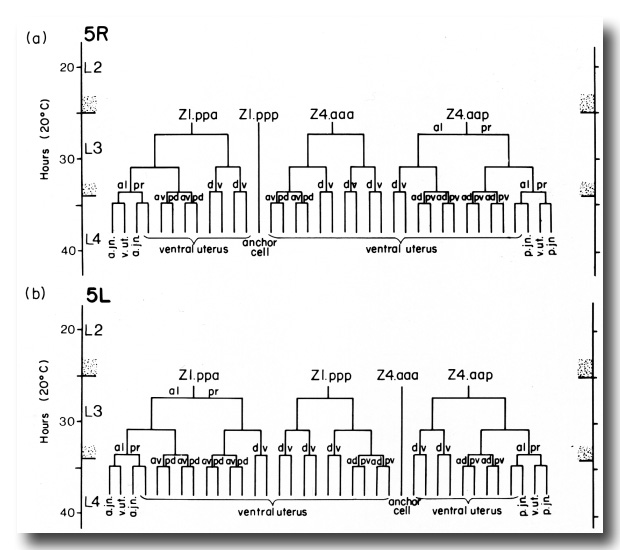 Figure 10. Alternate lineages of hermaphrodite lineage. Z1.ppa, Z1.ppp, Z4.aaa, and Z4.aap follow one lineage from the 5R configuration (a) and another lineage from the 5L configuration (b). The two alternate lineages are related by twofold rotational symmetry, a. jn., anterior spermathecal-uterine junction; p. jn., posterior spermathecal-uterine junction; v. ut., ventral uterus.
Figure 10. Alternate lineages of hermaphrodite lineage. Z1.ppa, Z1.ppp, Z4.aaa, and Z4.aap follow one lineage from the 5R configuration (a) and another lineage from the 5L configuration (b). The two alternate lineages are related by twofold rotational symmetry, a. jn., anterior spermathecal-uterine junction; p. jn., posterior spermathecal-uterine junction; v. ut., ventral uterus.
The difference in the ventral uterine lineage in 5L and 5R worms provides the only example of variability in the hermaphrodite lineage of Z1 and Z4. The 5R and 5L configurations differ only in the positions of two cells, but the lineages of four cells are affected. Figure 11 shows diagrammatically how the ancestry of cells in specific locations in the ventral uterus differs depending on whether the 5R or the 5L pathway is followed.
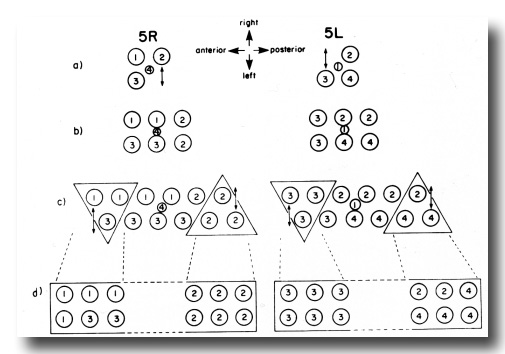 Figure 11. Comparison of development of the ventral uterus from 5R and 5L primordia. Dorsal view. Cell 1, Z4.aaa; cell 2, Z4.aap; cell 3, Z1.ppa; cell 4, Z1.ppp. Descendants of these cells are also marked by the same number. (a) Position of cells 1, 2, 3, and 4 in 5R and 5L primordia. 2 divides left-right from 5R (arrow), but 3 divides left-right from 5L (arrow). (b) In both cases, symmetry is restored. (c) One round of divisions later. The left row of cells has shifted posteriorly somewhat with respect to the right row of cells. Cells divide left-right and restore bilateral symmetry. The cells not included in triangles divide dorso-ventrally in the next round of divisions, and are therefore left out of the next figure. (d) Similar structures arise from 5R and 5L primordia, but the ancestry of cells that occupy equivalent positions in the structure is different.
Figure 11. Comparison of development of the ventral uterus from 5R and 5L primordia. Dorsal view. Cell 1, Z4.aaa; cell 2, Z4.aap; cell 3, Z1.ppa; cell 4, Z1.ppp. Descendants of these cells are also marked by the same number. (a) Position of cells 1, 2, 3, and 4 in 5R and 5L primordia. 2 divides left-right from 5R (arrow), but 3 divides left-right from 5L (arrow). (b) In both cases, symmetry is restored. (c) One round of divisions later. The left row of cells has shifted posteriorly somewhat with respect to the right row of cells. Cells divide left-right and restore bilateral symmetry. The cells not included in triangles divide dorso-ventrally in the next round of divisions, and are therefore left out of the next figure. (d) Similar structures arise from 5R and 5L primordia, but the ancestry of cells that occupy equivalent positions in the structure is different.
A uterine lumen begins to form during the last divisions of the uterine lineage. At this stage, the uterine cells are cuboidal with round nuclei and clear cytoplasm (figure 7). During the latter half of L4, the uterine cells become very thin. During this differentiation of the uterine epithelium, the uterine muscle cells, arising from a different lineage (Sulston and Horvitz, 1977), become attached to the uterus. The uterus begins to undergo massive distortion due to contractions, so the individual cells have not been traced through this period.
(d) Spermathecal-uterine junction lineage. Six cells contribute to the formation of the anterior, and six to the posterior, spermathecal-uterine junction. Their identities are indicated in figure 3 and figure 10. The adult junction consists of a junctional core that is surrounded by the nuclei of four cells (figure 12).
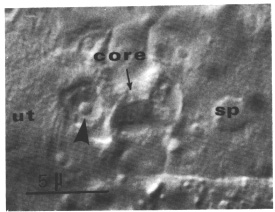 Figure 12.Spermathecal-uterine junction; Nomarski optics. This focal plane shows the junctional core and the nucleus (arrowhead) of one of the two cells that appear to form the core. ut, uterus; sp, spermatheca.
Figure 12.Spermathecal-uterine junction; Nomarski optics. This focal plane shows the junctional core and the nucleus (arrowhead) of one of the two cells that appear to form the core. ut, uterus; sp, spermatheca.
Two cells combine to make the junctional core. Because these two cells arise in what otherwise would be a spermathecal lineage, their identities are shown in figure 9. During L4, these two cells enlarge to form a plug between the lumens of the uterus and the spermatheca. During the L4 to adult molt, they differentiate into the core of the junction. The nuclei protrude into the uterine lumen as the main bulk of their cytoplasm elongates and takes on the shape of the junction's core (figure 12). These two nuclei are present until the first fertilized egg squeezes through the junction into the uterus. Their subsequent fates have not been determined.
The four other cells that contribute to the junction arise from what otherwise would be uterine lineages. These cells separate themselves from the developing uterus with which they had been associated and surround the junctional core to complete formation of the junction.
(e) Anchor cell. The anchor cell occupies a midventral position in the gonad during L3. The vulval precursor cells are located in the hypodermis underlying the gonad and are centered around the anchor cell. (See Sulston and Horvitz, 1977, for a more complete account of vulva formation.) As invagination of the developing vulva begins, the anchor cell assumes a position at its apex. During invagination, the anchor cell appears round and exhibits a prominent nucleolus and a granular cytoplasm. Once the invagination is complete, small vacuoles appear in the anchor cell cytoplasm ventral to the nucleus. The anchor cell then elongates, and its nucleus moves off center, leaving a cytoplasmic membrane spanning the vulval orifice. The anchor cell seems to be missing in the adult, but its fate is not known.
The Male Z1-Z4 Lineage (See figure 13)
Early Mitotic Period
In males, the early period of divisions generates 10 cells by early L2. As in hermaphrodites, Z1 and Z4 begin to divide about halfway through L1, and Z1.a and Z4.p occupy tip positions in the primordium, while Z1.p and Z4.a lie ventrally to the germ cells. Shortly after this first division, a fundamental difference in symmetry emerges between hermaphrodite and male developing gonads. The first indication of this difference is the anterior migration of Z4.a which disrupts the twofold rotational symmetry of the male primordium (figure 14). As Z4.a pushes forward, Z1.p displaces Z1.a at the anterior end of the primordium. The position of Z1.a changes from anterior to Z1.p, to dorsal to Z1.p, and then to a more posterior dorsal position (figure 14b). Cells Z1.a and Z4.p do not divide again in the male. As the male gonad elongates, Z1.a moves posteriorly along the dorsal margin of the primordium, until it lies just anterior to Z4.p at the posterior or distal tip of the gonad. These two cells become the male distal tip cells. In one animal, Z1.a did divide in the hermaphrodite fashion, but both daughter cells subsequently moved posteriorly to the distal tip to join Z4.p. In this case, Z1.aa and Z1.ap both acquired distal tip cell character; neither divided again, and both exhibited the small, oval nucleus which is characteristic of distal tip cells in males.
 Figure 13. One of the two alternate male lineages of gonadal somatic precursor cells Z1 and Z4. In the other lineage, Z4.aaa and Z1.paa exchange lineages so that Z4.aaa becomes the linker cell, and Z1.paa gives rise to 10 vas deferens cells and one seminal vesicle cell. Divisions give rise to an anterior daughter and a posterior daughter unless otherwise indicated. The fates of descendants are shown either individually or in groups: dtc, distal tip cell; lc, linker cell; s.v., seminal vesicle.
Figure 13. One of the two alternate male lineages of gonadal somatic precursor cells Z1 and Z4. In the other lineage, Z4.aaa and Z1.paa exchange lineages so that Z4.aaa becomes the linker cell, and Z1.paa gives rise to 10 vas deferens cells and one seminal vesicle cell. Divisions give rise to an anterior daughter and a posterior daughter unless otherwise indicated. The fates of descendants are shown either individually or in groups: dtc, distal tip cell; lc, linker cell; s.v., seminal vesicle.
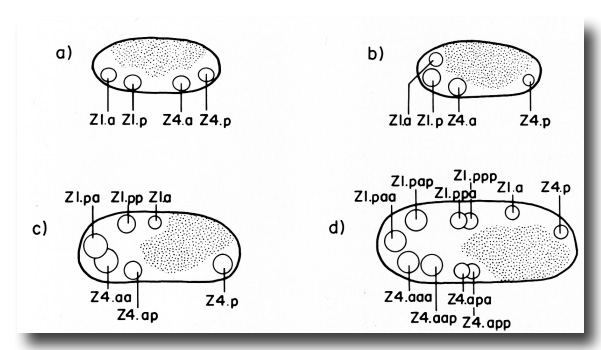 Figure 14. Spatial arrangement of male Z1 and Z4 descendant nuclei during the early mitotic period. All diagrams are lateral views with anterior to the left and dorsal above. The space occupied by Z2 and Z3 descendants is depicted by stippling, (a) position of nuclei just after the first division of Z1 and Z4, (b) position of same nuclei after migration has started, (c) and (d) positions of nuclei after subsequent rounds of divisions. Note that Z1.a moves along the dorsal margin of the gonad toward the posterior pole.
Figure 14. Spatial arrangement of male Z1 and Z4 descendant nuclei during the early mitotic period. All diagrams are lateral views with anterior to the left and dorsal above. The space occupied by Z2 and Z3 descendants is depicted by stippling, (a) position of nuclei just after the first division of Z1 and Z4, (b) position of same nuclei after migration has started, (c) and (d) positions of nuclei after subsequent rounds of divisions. Note that Z1.a moves along the dorsal margin of the gonad toward the posterior pole.
Z1.p and Z4.a undergo two rounds of divisions to generate a cluster of eight somatic cells at the anterior tip of the developing gonad. The first round is asymmetrical in that the anterior daughters are larger than the posterior daughters. These daughters all divide symmetrically in the second round. The eight resulting daughters form the male somatic primordium (figure 15).
 Figure 15. Male somatic primordium; Nomarski optics. The linker cell (arrow) occupies the anterior tip of the primordium. Posterior to the linker cell is a large vas deferens precursor cell (3) and posterior to that are two smaller seminal vesicle precursor cells (1 and 2). This anterior to posterior arrangement is invariant. However, since the positions of the three vas deferens precursor cells, and of the four seminal vesicle precursor cells, are variable around the long axis of the developing gonad, the numbered cells cannot be lineally identified unless their lineage had been followed from the L1 stage.
Figure 15. Male somatic primordium; Nomarski optics. The linker cell (arrow) occupies the anterior tip of the primordium. Posterior to the linker cell is a large vas deferens precursor cell (3) and posterior to that are two smaller seminal vesicle precursor cells (1 and 2). This anterior to posterior arrangement is invariant. However, since the positions of the three vas deferens precursor cells, and of the four seminal vesicle precursor cells, are variable around the long axis of the developing gonad, the numbered cells cannot be lineally identified unless their lineage had been followed from the L1 stage.
The male somatic primordium exhibits a variability in the positions of two cells. Either Z1.paa becomes the linker cell, and Z4.aaa becomes a vas deferens precursor cell, or vice versa (figure 16). The linker cell occupies the anterior tip position in the developing male gonad. The linker cell appears to serve a leader function during male gonadogenesis, preceding the developing gonad throughout its elongation. Three vas deferens precursor cells lie just posterior to the linker cell. The positions of these cells around the anterior-posterior axis are not fixed for any of the cells, and are interchangeable among the cells as they enlarge during L2. Four seminal vesicle precursor cells lie just posterior to the anterior block of the three cells described above. The positions of these four cells are also not fixed around the anterior-posterior axis as they enlarge during L2. The germ line component of the developing male gonad lies posterior to the somatic primordium.
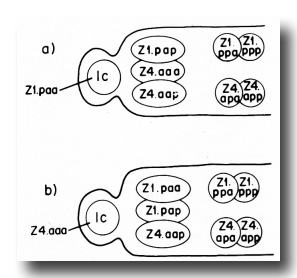 Figure 16. Alternate male somatic primordia. In (a) Z1.paa has become the linker cell (1c), whereas in (b) Z4.aaa has become the linker cell. Thus, Z1.paa and Z4.aaa have alternate fates.
Figure 16. Alternate male somatic primordia. In (a) Z1.paa has become the linker cell (1c), whereas in (b) Z4.aaa has become the linker cell. Thus, Z1.paa and Z4.aaa have alternate fates.
Amitotic Period
During the second larval stage, the cells of the somatic primordium grow significantly in size, but they do not divide. Growth of the developing gonad proceeds anteriorly by continuous divisions of the germ line cells. No posterior elongation is apparent. The developing gonad elongates from 25 to about 65-70 micrometer long during this period.
Late Mitotic Period
Seven somatic cells generate a total of 53 cells during the late mitotic period of the male. Divisions begin at the end of L2 continue through L3, and finish shortly after the L3-L4 molt. The cells gradually assume their adult appearance during L4. The somatic structures of the male include the seminal vesicle which holds mature sperm and the vas deferens which provides a passage for the sperm to the exterior via the cloaca (figure 17).
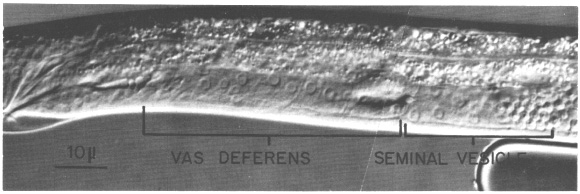 Figure 17. Male somatic structures in late L4 stage; Nomarski optics. Anterior is to the right, and dorsal is above.
Figure 17. Male somatic structures in late L4 stage; Nomarski optics. Anterior is to the right, and dorsal is above.
(a) Seminal vesicle lineage. Four cells in the male somatic primordium each contribute five cells to the seminal vesicle. The divisions of these precursor cells follow a simple pattern of four serial asymmetrical divisions that produce one larger and one smaller daughter in each round. The smaller cells do not divide further. The resultant 20 cells constitute the inner, apparently secretory layer of the seminal vesicle. Their cytoplasm becomes granular during L4 and small blebs appear at the lumenal surface as they mature. The outer layer of the seminal vesicle comprises three large, very thin cells. These cells are the most distal daughters of the three cells that give rise to the vas deferens (figure 13). They arise just proximal to the developing seminal vesicle. After these three cells arise, they begin to enlarge, flatten, and spread over the developing seminal vesicle to encapsulate it.
(b) Vas deferens lineage. Three cells in the male somatic primordium each contribute 10 descendants to the vas deferens and one descendant to the seminal vesicle. These three cells undergo a series of asymmetrical divisions, each of which generates one larger and one smaller daughter. In this lineage, the smaller cells each undergo one mitosis before differentiating. The vas deferens is a complex secretory tube consisting of a variety of cell types. Preliminary studies show at least three cell types based on morphological differences in secretory granules (Wolf and Kimble, unpublished observations). However, a detailed elucidation of the cellular anatomy is not complete, and study of the correlation between adult cells, cell types, and lineal descendants within the vas deferens is still in progress.
(c) Linker cell. During L4, the linker cell joins the vas deferens to two E cell descendants (Sulston and Horvitz, 1977) in the male tail. Just prior to the final molt, the linker cell undergoes the morphological changes typical of cell death. This cell death opens the passageway between the lumens of the vas deferens and the cloaca.
Discussion
Comparison of Gonadogenesis in Hermaphrodite and Male Worms
The two gonadal somatic progenitor cells present in the newly hatched worm can either follow a hermaphrodite or a male developmental pathway. If the hermaphrodite pathway is selected, the structures that are made display a twofold rotational symmetry, and 143 cells are generated. If the male pathway is selected, the structures made are asymmetrical, and only 56 cells are generated. The hermaphrodite and male developmental programs may be compared as follows.
(1) The arrangement and morphological appearance of the four cells in the gonadal primordium present in newly hatched worms is identical in both sexes. The somatic progenitor cells, Z1 and Z4, are located at the anterior and posterior tips of the primordium, respectively. The germ line progenitor cells, Z2 and Z3, occupy the region between Z1 and Z4. The primordium exhibits twofold rotational symmetry.
(2) Z1 and Z4 develop according to a temporal pattern that is essentially identical in hermaphrodites and males. During the second half of L1, Z1 and Z4 undergo a series of divisions that gives rise to 12 cells in the hermaphrodite and 10 cells in the male. During L2, these cells increase significantly in size without dividing further. By late L2, a somatic primordium takes shape due to migration and enlargement. During L3 and the first part of L4, the cells in this somatic primordium undergo extensive divisions. Cells that have stopped dividing differentiate morphologically to the characteristic adult appearance.
(3) The number and orientations of divisions in the early mitotic period are nearly the same in hermaphrodites and males. This lineage differs in the two sexes only by a single division of each somatic progenitor cell. Thus, in hermaphrodites, Z1.a and Z4.p undergo a division that usually does not occur in males. However, in one male, we observed that Z1.a divided in a hermaphrodite fashion. Since extra divisions of this sort have never been observed at any other point in the Z1-Z4 lineages of either sex, this extra division of Z1.a suggests that the hermaphrodite and male early lineages share a common program of control. For example, Z1 and Z4 may be programmed to divide in both sexes according to a single pattern with repression of one division in males.
An important difference in the early events of hermaphrodite and male gonad development occurs after the first division of Z1 and Z4. In the male, Z4.a begins its anterior migration, disrupting the twofold rotational symmetry of the primordium. This is the first morphological indication of the asymmetry that characterizes the rest of male gonad development. By contrast, hermaphrodites maintain the primordial symmetry throughout gonadogenesis.
(4) The three cells in the Z1-Z4 lineages that do not divide after the period of early divisions arise from equivalent positions in the early lineage trees and have similar development fates in hermaphrodites and males (figure 18). In both sexes, two of these cells become distal tip cells, and the third cell, either the anchor cell or linker cell, serves to join the gonadal lumen with the animal's exterior. The anchor and linker cells also share the characteristic of variable ancestry. Either Z1.ppp or Z4.aaa can become the anchor cell in hermaphrodites, and either Z1.paa or Z4.aaa can become the linker cell in males.
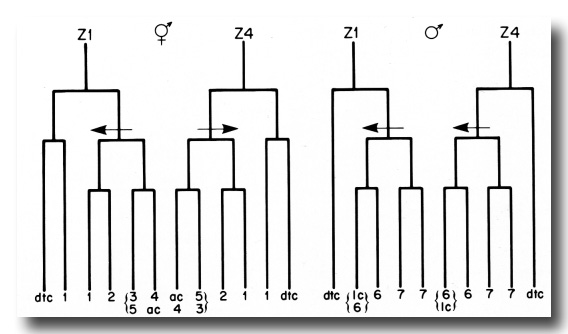 Figure 18. Comparison of the hermaphrodite and male Z1 and Z4 early division patterns. The arrows indicate asymmetric divisions, and point to the larger of the two daughters in each case. The numbers refer to the developmental fates of the cells that arise from the early divisions: 1, sheath-spermatheca lineage; 2, dorsal uterus-spermatheca lineage; 3, 4, and 5, ventral uterus lineages; 6, vas deferens lineage; 7, seminal vesicle lineage; dtc, distal tip cell; ac, anchor cell; lc, linker cell. See text for further explanation.
Figure 18. Comparison of the hermaphrodite and male Z1 and Z4 early division patterns. The arrows indicate asymmetric divisions, and point to the larger of the two daughters in each case. The numbers refer to the developmental fates of the cells that arise from the early divisions: 1, sheath-spermatheca lineage; 2, dorsal uterus-spermatheca lineage; 3, 4, and 5, ventral uterus lineages; 6, vas deferens lineage; 7, seminal vesicle lineage; dtc, distal tip cell; ac, anchor cell; lc, linker cell. See text for further explanation.
In hermaphrodites, the distal tips move away from the original primordium site, and the most proximal point, marked by the anchor cell and developing vulva, is located where the primordium had been in the L1. In males, the future proximal end moves away, led by the linker cell, and the distal tip is found at the primordium site. Thus, the polarity of the distal-proximal axis of the hermaphrodite is opposite that of the male with respect to the direction of elongation of the gonad. However, in both sexes the spatial and temporal polarity of gamete maturation is toward the somatic structures, and in this sense, they are equivalent. The distal tip cells and the proximal anchor or linker cell might be a reflection of the early establishment of the distal-proximal axes, or they themselves might function to establish the distal-proximal axes of the gonad.
(5) A homologous relationship can be drawn between individual hermaphrodite and male somatic structures by similarities in their positions, lineages, and functions. The most distal somatic structures in both sexes consist of thin, flat cells which encase maturing gametes: the sheath in hermaphrodites and the seminal vesicle in males. In both cases four cells in the somatic primordium each contribute five cells toward that structure, albeit through different lineage patterns. The male vas deferens and the hermaphrodite spermatheca are the next structures in line from distal to proximal. Since the spermatheca houses sperm, and can be considered a male component in the hermaphrodite, and since the lineages are very similar that give rise to these structures, they may be homologous structures. Electron microscopic studies have also revealed an ultrastructural similarity between the vas deferens and the spermatheca (Wolf and Hirsh, unpublished observations). The uterus has no obvious counterpart in the male lineage.
Comparison of gonadogenesis in hermaphrodites and males leads to the striking conclusion that similar developmental programs are followed to generate structures that differ in their properties of symmetry and terminal differentiation. Thus, the decision to make a hermaphrodite or male gonad probably pivots on a limited number of critical modifications in a fundamental program. This hypothesis is reminiscent of Ohno's hypothesis that the genetic control of mammalian sex differentiation is simple and depends on the presence or absence of testosterone during a particular period of development (Ohno, 1971). The genetic control of sex differentiation in C. elegans is also simple. After intensive searching for mutants that alter sex differentiation, only three genes have been identified that transform XX individuals into males (Hodgkin and Brenner, 1977), and only two genes have been identified that transform XO individuals into hermaphrodites (Nelson et al., 1978; Hodgkin, personal communication).
The temporal control of the decision to make either a male or a hermaphrodite gonad has been investigated in a temperature-sensitive transformer mutant that produces males in XX worms (Klass et al., 1976). The critical period of temperature sensitivity in this mutant extends from 3 hr before hatching to 12 hr after hatching (25°C). Thus, 12 hr after hatching is the first point in development at which a shift to restrictive temperature cannot cause any of the worms to make a male gonad. Since the first sign of sexual dimorphism occurs at about 9 hr (25°C) after hatching, the morphological divergence in the two programs may indicate a point of irreversible commitment to one developmental pathway.
Comparison of the Z1-Z4 Lineages to Other Lineages in C. elegans Lineage patterns.
Sulston and Horvitz (1977) discuss several standard lineage patterns that were observed in the nongonadal lineages. These patterns are also seen in the gonadal lineages. In fact, the entire male lineage can be described in terms of these basic patterns of division.
One of the simpler patterns is characterized by a stem cell repeatedly dividing asymmetrically to give rise to another stem cell and a differentiated cell that does not divide again. An archetypal example of stem cell logic is seen in the lineage of the seminal vesicle. Stem cell logic is also found in the early embryonic lineage of C. elegans (Deppe et al., 1978) and in the postembryonic lateral hypodermal lineages (Sulston and Horvitz, 1977).
A more complex type of stem cell pattern has been suggested from histological studies of neuroblast development in Drosophila (Poulson, 1950). In this variation, the stem cell gives rise to another stem cell and a "pre-differentiated cell" that will divide one more time before differentiating. The vas deferens lineage in C. elegans provides an example of this pattern. The spermathecal lineage of the hermaphrodite begins to divide according to this pattern, but then diverges from it. A similar pattern is seen in the ventral nerve cord lineage of C. elegans (Sulston and Horvitz, 1977). Thus, this pattern may represent a basic mechanism of increasing cell number in different tissues and different organisms.
The early lineage patterns of hermaphrodites and males represent a third basic, but more variable lineage type. A pattern identical to the male early lineage gives rise to each of the rays in the male tail, and similar patterns are seen in the lineages of the posterior lateral ganglia and the lumbar ganglia (Sulston and Horvitz, 1977). Sulston and Horvitz (1977) compare this type of pattern to an insect pattern (Lawrence, 1966) and suggest that it reflects a developmental program common to both organisms.
The standard patterns of cell division observed in C. elegans imply the existence of simple programs by which cell number is increased. These programs may or may not direct a differential segregation of developmental potential to the daughters that arise in the lineages as will be discussed under Mechanisms of Determination.
Temporal control of division. Z1 and Z4 follow a temporal pattern of divisions that is also found in other postembryonic lineages. This pattern is characterized by two periods of divisions separated by a period when no divisions occur. Two nongonadal lineages follow the same pattern (Sulston and Horvitz, 1977). The mesoblast cell, M, undergoes a series of divisions during the last half of L1 followed by a migration of certain of its early descendants during L2 (hermaphrodites) or L3 (males). These cells divide further in L3 to generate the sexual musculature of the vulva or the male tail. The P precursor cells of the ventral nerve cord follow a similar pattern in which a few of the descendants which arise in L1 divide again in L3.
The feature common to cells that divide first in L1 and then in L3 is their involvement in the development of the reproductive system. The function of this delay in development is open to speculation. A full elaboration of the sexual structures might be deferred until after the decision is made in L2 (Cassada and Russell, 1975) to bypass the dauer larval pathway and complete the sexually mature form. Fewer gonadal cells would be economical of the resources necessary to maintain the dauer larva until it finds a new source of nutrients. On the other hand, the L3 developmental delay may serve to ensure the coordinate development of the sexual tissues.
Mechanisms of Determination
Ancestry vs position. A fundamental question that arises out of lineage studies is how cells are led to diverge in their capacity for differentiation. Two mechanisms were proposed by Roux (1888). Cells might become committed to a particular pathway by "self-differentiation" (or cell ancestry) or by "correlative dependent differentiation" (or cell-cell interaction). If a cell is determined by ancestry, cell divisions must mediate a segregation of developmental potential to daughter cells. If a cell is determined by position, cell divisions might only serve to increase the number of cells. A third possibility is that both cell ancestry and cell position play significant roles in the determination of cells in development. For example, a segregation of cytoplasmic determinants during early cleavages in the embryo might restrict a cell's ability to differentiate to one subset of pathways, and cell position might convey to a cell which specific program it must follow among the available choices. Or, the determinants sequestered to one daughter rather than the other might allow that cell to respond differentially to external signals.
Invariance in cell lineages is regarded as a principle of nematode development, both classically (Boveri, 1899) and currently (Sulston, 1976; Sulston and Horvitz, 1977; Deppe et al., 1978). Such invariance only demonstrates that the developmental program is rigidly controlled. The inability of cells to alter their program after manipulation, such as isolation of blastomeres (e.g., Wilson, 1925) or ablation of neighboring cells (Sulston and Horvitz, 1977) shows that cells are irreversibly committed, but does not reveal how that commitment was established. In most lineages, the invariance observed in division pattern and cell fate also corresponds to an invariance in cell position. When both lineage and position of descendant cells are invariant, the influence of cell ancestry and/or position on cell fate is extremely difficult to assess. However, the limited variability that is observed in essentially invariant developmental programs can be utilized to explore the significance of position and ancestry to cell determination.
The most common pattern of variability seen in the Postembryonic lineages of C. elegans involves two cells, each of which is capable of following one of two alternative lineages. The two cells occupy one of two alternative positions, but once a position is assumed, the cell follows a lineage pattern that corresponds to that position.
The nongonadal lineages provide several examples of such variability (Sulston and Horvitz, 1977). Two pairs of cells in the male tail exhibit alternative lineages correlated with position. Certain pairs of ventral cord precursor cells follow different lineages depending on their anterior-posterior position after migration into the cord from the left and right sides of the worm. In addition, two cells in the male gonadal lineage exhibit alternative lineages. The more anterior of the pair of cells becomes the linker cell, whereas the more posterior follows a typical vas deferens lineage pattern.
In hermaphrodites, a more complex situation is found. The positions of only two cells (Z1.ppp and Z4.aaa) are variable, but the lineages of four cells are altered (figure 10). Concurrent with the establishment of the hermaphrodite somatic primordium at the end of L2, the four cells that give rise to the ventral uterus assume one of two configurations. These four cells, as a group, follow one of two alternative lineage patterns which corresponds to the configuration in the individual primordium. This group phenomenon suggests that neighboring cells in some way can influence each other's fates.
Another explanation of the hermaphrodite variability might be that the two somatic progenitor cells can randomly assume the anterior or posterior positions during the embryonic formation of the four-celled gonadal primordium. If only one of the somatic progenitor cells were capable of producing an anchor cell, for example, and if that cell could become either Z1 or Z4, the observed variability would result.
A third explanation of the 5R and 5L alternatives might have been that a random population consists of two subpopulations, one programmed to follow the 5R pattern and one programmed to follow the 5L pattern. This cannot be the case because these programs are not clonally inherited; individuals whose developmental pattern was recorded as 5L or 5R and were then cloned gave rise to progeny that followed both 5L and 5R patterns.
Thus, two hypotheses remain that might explain the unique variability observed in the hermaphrodite Z1-Z4 lineage. Either two somatic progenitor cells are irreversibly committed during embryogenesis and can interchangeably become Z1 or Z4, or the interaction of the cells in the somatic primordium affects their subsequent lineage pattern. It may be possible to distinguish between these two explanations by laser ablation of Z1 or Z4 in a large number of animals. If Z1 and Z4 are irreversibly committed cells, an anchor cell should arise in approximately one half of such experimental animals. If Z1 and Z4 are not irreversibly committed, an alteration in the development of the remaining cell may be seen. If some form of regulation occurs and cell interaction proves to be the most reasonable explanation for the variability in the lineages of four of the cells in the somatic primordium, one might postulate that the rearrangement of cells to form a somatic primordium represents a change in position for all the cells involved, and that cell-cell interaction might influence the developmental fate of all the cells concerned.
Coordinates and developmental fates. Whether two daughters are determined because they have acquired a specific cytoplasm or a specific position, some kind of coordinate system is an absolute requirement for information to be conveyed differentially to daughter cells. An anterior daughter might follow a different pathway from its posterior sibling due to a differential segregation of cytoplasmic determinants to the two daughters. However, the difference might just as reasonably be due to a different environment imposed on the daughter cells, whether that is mediated through interaction with its neighbors or an externally imposed gradient. Thus, lineage data do not reveal the mechanisms by which the fate of one daughter becomes different from the fate of its sibling. Yet, lineage data demonstrate that the fate of a cell does correspond to the spatial relationship in which the siblings arise, and therefore, shows that the coordinate exists and is significant to the fate of the daughter cells.
The male Z1-Z4 lineage provides evidence that at least the last half of male gonadal development proceeds according to its own coordinate system rather than the organismic coordinate system. The late divisions of the male begin just before the gonad makes its 180°turn. Since there is some variability in the correlation between when a cell divides and when the gonad makes its bend, a cell may divide before the turn, in the turn, or after the turn. The daughter nearer the leading or proximal edge would be an anterior daughter in the first case, a dorsal daughter in the second, and a posterior daughter in the last case. Yet, that daughter does not vary with respect to further divisions or differentiation. Thus, distal-proximal coordinates along the gonadal axis seem to be more relevant to these divisions than the coordinates of the worm. Since the initial growth of the male gonadal primordium is only anterior and never posterior, it seems likely that the organismic coordinate system influences this first developmental step in the male. The twofold rotational symmetry exhibited by the hermaphrodite gonad does not contradict the idea that the gonadal coordinate system may be independent of the organismic coordinate system.
One principle that has emerged from the information on position and cell fate in the lineages of Z1 and Z4 is that the symmetry of the structure in which a particular division takes place is correlated with the symmetrical relationship between the fates of Z1 descendants and the fates of Z4 descendants (figure 18). In both hermaphrodites and males, the fates of the daughters of the first division follow the twofold rotational symmetry of the four-celled gonadal primordium. Thus, Z1.a is equivalent to Z4.p, and Z1.p is equivalent to Z4.a. A comparison of the fates of Z1 descendants with the fates of Z4 descendants shows that, in hermaphrodites, cells that are equivalent in developmental fate occupy positions that are related by twofold rotational symmetry (figure 18). The morphology of the hermaphrodite developing gonad also retains the twofold rotational symmetry. By contrast, the male developing gonad becomes morphologically asymmetrical soon after the first division. And, in males, equivalent cells in the Z1 and Z4 lineages are related asymmetrically along the anterior-posterior axis after the first division (figure 18).
In hermaphrodites, the only cells in the somatic primordium that are not positioned according to twofold rotational symmetry have lineages that are not related to each other by twofold rotational symmetry. So, Z1.ppa is not lineally equivalent to Z4.aap, and Z1.ppp is not equivalent to Z4.aaa. However, these four cells can assume one of two configurations, 5R and 5L, that are related by twofold rotational symmetry, and the lineages followed by the four cells in 5L are related to the lineages of the four cells in 5R by twofold rotational symmetry.
The significance of the correlation observed between the morphological symmetry displayed by the developing gonad and the developmental symmetry exhibited by the fates of Z1 and Z4 descendants is purely a matter of speculation at the present time. The correlation might be coincidental, it might be a reflection of two independent responses to an underlying coordinate system, or it might involve a direct cause and effect relationship.
A second principle that emerges from the Z1-Z4 lineages is that no structure (e.g., uterus, anterior spermatheca, vas deferens) develops as a cell clone. Clonal development is defined by two criteria (Crick and Lawrence, 1975). First, a cell must contribute all its descendants to a single structure. Second, a structure must consist of only the descendants of a single clone. In the development of the gonadal somatic structures, each structure consists of descendants from more than one cell in the somatic primordium, and in most cases, each cell in the somatic primordium contributes to more than one structure. Such nonclonal development of structures is also typical of organisms whose lineages were followed in classical studies. For example, most structures in the trochophore larvae of the annelid Nereis (Wilson, 1892) and the mollusk Crepidula (Conklin, 1897) -apical rosette, prototroch, gut- are derived from descendants of all four progenitor cells that arise from the first two divisions of the egg.
In conclusion, the Z1-Z4 lineages are particularly advantageous for addressing certain key developmental questions:
(1) The unique variability in the hermaphrodite lineage strongly suggests that cell-cell interaction affects cell fate during gonadogenesis. Alternatively, the two somatic progenitor cells might be irreversibly committed during embryogenesis and assume the Z1 or Z4 position interchangeably in the gonadal primordium. The laser ablation of Z1 or Z4 will test the commitment of the unablated cell and therefore may distinguish between the two models.
(2) The two developmental programs available to Z1 and Z4 raise questions about how the genetic control of the programs is organized, and how differences in symmetry are established. The genetic control is accessible since C. elegans is amenable to mutational analysis and since gonadogenesis defective mutants can be obtained (Hirsh and Vanderslice, 1976). However, the necessity for a selection method for mutants that specifically affect morphogenesis has become apparent (Kimble, 1978). The establishment of symmetry is a much more elusive problem, but the morphological change in symmetry that is reflected in the behavior of four cells in the male may provide a key to how that change is mediated.
(3) The significance of nonclonal development of the gonadal somatic structures may also be approachable in this system. One guess is that cell-cell interaction is important to the evolution of complexity during development. It should be possible to alter the composition of the cells of the somatic primordium by laser ablation so that only one precursor cell is left for a particular structure. If cell interaction between two precursor cells is critical to differentiation, such a change should cause a change in the pattern of differentiation of the precursor cell.
The Z1-Z4 lineages provide a model of morphogenesis and differentiation that is sufficiently complex so the principles involved will probably be applicable to the development of most higher eukaryotes. Yet, the system is simple enough that the behavior of individual cells can be studied as they proceed through the sequential steps leading to the adult form. Our current goal is to understand the extents to which ancestry and cell interaction play a role in the determination of individual cells. Ultimately, we hope to exploit this system to elucidate the genetic control of the cells' progression through each step in gonadogenesis.
Acknowledgments
We thank John Sulston for generously sharing his lineage technique with us, and for his supportive and stimulating communications during the progress of this work. We also thank Scott Emmons, Bill Wood, and Larry Gold for many thought-provoking conversations.
This work was supported by Public Health Service Grant No. GM 19851 from the National Institutes of General Medical Sciences and in part by BRSG Grant RR0713-11 awarded by the Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health. DH was the recipient of a Public Health Service Research Career Development Award (GM 70465) and JK was an NSF predoctoral fellow.
References
ALBERTSON, D. G., SULSTON, J. E., and WHITE, J. G. (1978). Cell cycling and DNA replication in a mutant blocked in cell division in the nematode Caenorhabditis elegans. Develop. Biol. 63, 165-178.
BOVERI, T. (1899). "Die Entwicklung von Ascaris megalocephala mit besonderer Rucksicht auf die Kernverhaltnisse." Gustav Fischer, Jena.
BRENNER, S. (1975). The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
CASSADA, R. C., and RUSSELL, R. L. (1975). The dauer-larva, a postembryonic developmental variant of the nematode Caenorhabditis elegans. Develop. Biol. 46, 326-342.
CHITWOOD, B. B., and CHITWOOD, M. B. (1950) "Introduction to Nematology." University Park Press, Baltimore.
CONKLIN, E. G. (1897). The embryology of Crepidula. J. Morphol. 13, 1.
CRICK, F. H. C., and LAWRENCE, P. A. (1975). Compartments and polyclones in insect development. Science 189, 340-347.
DEPPE, U., SCHIERENBERG, E., COLE, T., KRIEG, C., SCHMITT, D., YODER, B., and VON EHRENSTEIN, G. (1978). Cell lineages of the embryo of the nematode Caenorhabditis elegans. Proc. Nat. Acad. Sci. USA 75, 376-380.
HIRSH, D., OPPENHEIM, D., and KLASS, M. (1976). Development of the reproductive system of Caenorhabditis elegans. Develop. Biol. 49, 200-219.
HIRSH, D., and VANDERSLICE, R. (1976). Temperature-sensitive developmental mutants of Caenorhabditis elegans. Develop. Biol. 49, 220-235.
HODGKIN, J. A., and BRENNER, S. (1977). Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics 86, 275-287.
KIMBLE, J. (1978). "The Post-embryonic Cell Lineages of the Hermaphrodite and Male Gonads in Caenorhabditis elegans." Ph.D. Thesis, University of Colorado.
KLASS, M., WOLF, N., and HIRSH, D. (1976). Development of the male reproductive system and sexual transformation in the nematode Caenorhabditis elegans. Develop. Biol. 52, 1-18.
LAWRENCE, P. A. (1966). Development and determination of hairs and bristles in the milkweed bug, Oncopeltus fasciatus (Lygacidae, Hemiptera). J.
Cell Sci. 1, 475-498.
LEES, A. D., and WADDINGTON, C. H. (1942). The development of the bristles in normal and some mutant types of Drosophila melanogaster. Proc. Roy. Soc. Ser. B 131, 87-110.
NELSON, G., LEW, K. K., and WARD, S. (1978). Inter-sex, a temperature-sensitive mutant of the nematode Caenorhabditis elegans. Develop. Biol. 66, 386-409.
OHNO, S. (1971). Simplicity of mammalian regulatory systems inferred by single gene determination of sex phenotypes. Nature (London) 234, 134-137.
PAI, S. (1927). Lebenszyklus der Anguillula aceti. Ehrbg. Zool. Anz. 74, 257-270.
POULSON, D. F. (1950). Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster Meigen. In "Biology of Drosophila" (M. Demerec, ed.), pp. 168-274. Hafner, New York.
ROUX, W. (1888). Beitrage zur Entwicklungsmechanik des Embryo. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 114, 113.
SULSTON, J. E. (1976). Post-embryonic development in the ventral cord of Caenorhabditis elegans. Phil. Trans. Roy. Soc. London B 275, 287-297.
SULSTON, J. E., and HORVITZ, H. R. (1977). Postembryonic cell lineages of the nematode, Caenorhabditis elegans. Develop. Biol. 56, 110-156.
WILSON, E. B. (1892). The cell lineage of Nereis. J. Morphol. 6, 362.
WILSON, E. B. (1925). "The Cell in Development and Heredity," 3rd ed. Macmillan, New York.
|