Abstract - Materials and Methods - Introduction - Results - Discussion - References
Abstract
Serial-section electron microscopy has been used to reconstruct the cellular architecture of the posterior nervous system of the nematode Caenorhabditis elegans. Each of 40 neurons in the tail of the adult hermaphrodite can be reproducibly and unambiguously identified by a set of
morphological features, including cell body position, fiber geometry and
size, and staining properties. A complete list of synapses has been
assembled for 2 isogenic animals, and these lists are compared with a third
isogenic animal reconstructed by White et al. (1986). The set of neurons
and their pattern of synaptic interactions is simple and reproducible. Most
of the cells are involved in sensory transduction or in local signal
processing to relay signals via a few interneurons to motoneurons and
thence to body muscles. Because the tail neurons are well separated and
fairly reproducible in position, the hermaphrodite tail lends itself to
laser-ablation studies of sensory processing (cf. Chalfie et al., 1985).
Most of the synapses in the tail are concentrated in the preanal ganglion.
Among the approximately 150 synapses there, about 85% are dyadic chemical synapses. The dyadic synapses are involved in reproducible patterns that have several interesting features. Most neurons synapse onto a few preferred pairs of target cells, in patterns that suggest a combinatorial model of synapse specification that may be open to genetic analysis. Furthermore, most dyadic contacts A->B,C fit a pattern in which the 2 postsynaptic partners are involved elsewhere in unidirectional synapses B -> C. Thus, the dyadic synapse may serve to diverge sensory signals into parallel pathways, which then reconverge. This divergence/reconvergence pattern eventually directs processed sensory signals to the ventral cord interneurons PVCL and PVCR. About 80-90% of the synapses fall into repeated classes of synapses. Many of the remaining synapses are widely scattered and irreproducible from one animal to the next. Some of these contacts may be developmental mistakesreflecting a degree of "noise" in synapse specification(Waddington, 1957).
Received Feb. 19, 1990; revised Aug. 16, 1990; accepted Aug. 17, 1990. This work was supported by a Sloan Foundation Neurosciences grant to R.L.R.,
by U.S. Public Health Service Grants NS09654 and NSI3749 to R.L.R., NSI5909
to D.H.H., NS07512 to M. V.L. Bennett, and by U.S. Public Health Service Training
Grant GM02031 to D.H.H. We wish to thank John White, John Sulston,
Nicol Thomson, and Lois Edgar for extensive cooperation in the identification of
cells and for sharing many results with us before publication. We also thank
Randle W. Warc for an introduction to serial-section electron microscopy. Finally,
we are indebted to M. V. L. Bennett for his continuing interest in these
studies.
Correspondence should be addressed to David H. Hall, Department
of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461.
a Present address: Department of Biological Sciences, University of Pittsburgh,
Pittsburgh, PA 15260.
Copyright (c) 1991 Society for Neuroscience 0270-6474/91/010001-22$03.00/0
Materials and Methods
Fixation and embedding. Caenorhabditis elegans (var. Bristol, strain N2) were grown monoxenically at 20°C in Petri plates containing nematode growth medium (NGM) agar preseeded with Escherichia coli strain OP50 (Brenner, 1974). Synchronous populations (initiated during a 45-min hatching period) were grown at 20°C for 50 hr, at which point they had just begun to lay eggs (Byerly et al., 1976). Subsequent fixation and embedding steps were as described by Ware et al. (1975). Briefly, animals were rinsed off plates and washed several times in S medium (Brenner, 1974), anesthetized for 15 min in 0.5% 1-phenoxy-2-propanol in 0.1 M cacodylate buffer (pH, 7.4), and fixed in 2% osmium tetroxide in 0.1 M cacodylate buffer (pH, 7.4) for 1-4 hr at 23°C. The tails were then cut off with an X-acto blade and stained for 45 min in 1% uranyl acetate, 0.05 M maleate buffer (pH, 6.1). After dehydration through a graded series of ethanol and acetone, the cut pieces were embedded in Epon-Araldite.
The lack of an aldehyde fixation resulted in a rather washed-out appearance, which aided the serial reconstruction without preventing the identification of synaptic contacts. However, for some of the micrographs shown in Figure 8, aldehyde-fixed tissues were used to allow a more representative view of the cytoplasmic features of chemical synapses.
Sectioning and reconstruction. For microtomy, individual tail pieces were cut out of a flat mold and mounted on support blocks with epoxy cement. Serial sections were cut perpendicular to the body axis with a diamond knife on a Sorvall MT2B ultramicrotome at a thickness of 50-60 nm. Sections were collected in strips on slot grids (15-30 sections per grid). Continuity was very good, as the loss of sections was generally 3% or less. Typical series were 1000-2000 sections long.
Sectional series were used to derive general information from 8 adult animals (Hall, 1977). All of the general features described, down to the level of cell numbers and positions, have been confirmed in 4 or more animals. More detailed information, including the identification of cell types by fiber tracing, were principally derived from 2 long series, B126 and B136, which are essentially complete for all of the posterior ganglia, for their associated commissures, and for the included synapses. In addition, many of the reported morphological findings were confirmed or extended by Lois Edgar in an independently reconstructed series (L. Edgar, personal communication) and by White et al. (1986) in a reconstructed series. Repeated reexamination of our series and those of White et al. were required to settle a few difficult points, particularly where several fibers pass together through tortuous commissures. These comparisons served to double-check the identities and features of posterior cells, but more importantly, they allowed many anteriorly projecting processes to be identified as distal parts of already described anterior cells (White et al., 1986). There are several minor changes in axon identifications compared to those previously reported (Hall, 1977).
The reconstruction of long fiber tracts (500-800 sections) was facilitated by serial-section cinematography, using methods and equipment designed by R. Ware (Levinthal and Ware, 1972; Ware et al., 1975). The ganglia were reconstructed from high-magnification prints of every section; this was required to catalog every synapse and to trace difficult commissures. Microscopy was done on Zeiss EM9 and Philips 300 electron microscopes.
Nomenclature. We have adopted the cell-naming system of White et al. (1986). Bilateral pairs of tail neurons are referred to together by a 3-letter name and separately by the same name followed by L or R to indicate the left or right member of the pair. Ventral cord motoneurons have 2-letter names, followed by a number that corresponds to their place in order along the ventral cord (see Table 1 for a list of abbreviations and Table 2 for a summary of cell types and nomenclature).
Our characterizations of neurons as motoneurons, sensory neurons, or interneurons were based only on our anatomical findings, except for the PLM sensory neurons, where reported studies using laser microbeam ablation and mutants have provided direct functional evidence for the mediation of a touch response (Chalfie and Sulston, 1981; Chalfie et al., 1985). Among all other neurons, those with reproducible synaptic outputs onto muscle cells were called motoneurons, those with apparent sensory specializations were called sensory neurons, and all others were called interneurons, provided that they involved both pre- and post-synaptically somewhere in the animal (not necessarily in the tail). One limitation of this scheme is that we may have misclassified sensory neurons whose sensory specializations were not obvious. A few neurons have properties combining those of 2 described classes; such instances are dealt with specifically in the text.
Among the ascribed interneurons, we have designated as "major" and "minor" those with many and few synaptic contacts, respectively. However, because the physiological strength of the synapses cannot be determined with present methodology, these designations do not rule out the possibility that "minor" interneurons, with only infrequent synaptic interactions, may nonetheless make significant contributions to the animals behavior.
Enumeration of synapses. For the cataloging of synapses, 2 entire sectional series described in this paper (B126 and B136) were used. In each series, occasional sections were missing, and occasional sections were relatively poor in quality, but there were no serious gaps in either series. A primary list of synapses was made for each animal, noting the position of each synapse by section number. Chemical synapses included in this list showed presynaptic specializations for 3-10 successive sections (an electron-dense button or rod along the cytoplasmic face of the presynaptic membrane and increased membrane density, accompanied by a cluster of vesicles). A secondary list of possible chemical synapses was made where 2 or 3 sections adjoining missing or damaged sections displayed indications of a presynaptic specialization. The pattern of interactions in each secondary list was not noticeably different from that in the primary lists; hence, the 2 lists were combined in each case. For B126 and for B136, the combined lists contained 159 and 144 synapses, respectively. These lists include every morphologically identifiable posterior synapse in the corresponding animal, exclusive of neuromuscular junctions (NMJs) in the dorsal cord. Synaptic contacts in the dorsal nerve cord were enumerated separately in an essentially complele series from B136.
Gap junctions (electrical synapses) tend to be rather small and variable, depending upon section angle. Thus, their identification is more subjective and difficult to codify. It is less certain that we have enumerated all gap junctions in the posterior nervous system in either B126 or B136.
Introduction
The small soil nematode Caenorhabditis elegans has received considerable attention as a model organism in which a genetic approach can be brought to bear on questions of eukaryotic development (Hedgecock et al., 1987, 1990; Desai et al., 1988; Wood, 1988; Chalfie and Au, 1989). In part, this attention is based on the relative genetic manipulability afforded by C. elegans' self-fertilizing, hermaphroditic mode of reproduction (Brenner, 1974; Herman and Horvitz, 1980), and, in part, it is based on the fact that C. elegans has a small and reproducible number of somatic cells whose lineage histories have been completely described from the fertilized zygote to the adult (Sulston, 1976; Sulston and Horvitz, 1977; Deppe et al., 1978; Sulston et al., 1983). The nervous system of C. elegans is of great developmentalinterest. Its functional organization must depend not only on general aspects of development, such as tissue-specific differentiation or selective adherence of cells of like tissue type, but also on differentiation of a considerable variety of specific neuronal types and especially on the establishment of highly specific patterns of outgrowth and synaptic interconnection among these types (White et al., 1983; Chalfie et al., 1985; Hedgecock et al., 1987, 1990; Chalfie and White, 1988). C. elegans mutants can remain viable despite catastrophic lesions to the nervous system (Hedgecock et al., 1987, 1990; Hall et al., 1989), offering access to many essential developmental genes. Molecular studies in both vertebrates and invertebrates have shown significant homologies in early neural development (Hedgecock and Hall, 1990; Way, 1990); these homologies suggest that C. elegans mutants may uncover basic developmental mechanisms of broad importance. As a necessary prerequisite to developmental study of the nervous system, previous reports have described parts of the adult C. elegans nervous system in considerable detail, including the anterior sensory cells (Ward et al., 1975; Ware et al., 1975), the pharynx (Albertson and Thomson, 1976), the ventral nerve cord (White et al., 1976), and most elaborately, the nerve ring (White et al., 1986). Analysis has been facilitated by the relative simplicity of the nervous system (302 neurons in total; White et al., 1986) and by its very limited physical size, which has made possible the complete reconstruction of portions of the nervous system by serial-section electron microscopy with series of, at most, a few thousand sections in length.
Here, we describe in detail the patterns of synaptic interaction observed in the tail of the adult hermaphrodite, cataloging all the synapses observed and establishing the unique identities of pre- and postsynaptic cells at most synapses. When combined with the data from White et al. (1986), our results allow inferences to be made about the degree of developmental reproducibility in synapse formation and about the probable relationships between bilaterally homologous cells. They also suggest interesting possibilities for the ways in which the observed synapses might serve to process sensory information.
Results
General anatomy of the posterior nervous system. Some of the general anatomical features of the C. elegans hermaphrodite tail are indicated schematically in Figure 1. The tail tapers to a fine tip posteriorly, with small lateral openings in the cuticle for the phasmids, a bilateral pair of sense organs, and a larger ventral opening for the anus (Fig. 1A). Dark-staining rectal cells interrupt the passage from the intestine to the anus. A sphincter muscle cell (Fig. 1A, SI) controls the rectal opening, while single large muscle cells operate to open the anus (depressor ani muscle; Fig. 1A, D) and to squeeze the posterior intestine (stomatointestinal muscle; Fig. 1A. SI). The ventral cord terminates at the preanal ganglion, which contains 13 loosely associated cell bodies (Fig. 1B). Slightly above and to the rear of this ganglion is the small dorsorectal ganglion, whose 3 cells are joined to the preanal ganglion by a pair of circumrectal commissures. Extending posteriorly from the preanal ganglion are a pair of ventrolateral commissures, which pass on either side of the anus to reach 2 bilaterally symmetrical lumbar ganglia, each containing 12 loosely associated neuronal cell bodies. Figure 1B shows a view from the right side, where only the rightward commissures and the right lumbar ganglion are evident. The dorsal cord terminates in the tail by trifurcating to form a pair of circumferential commissures, which enter the lumbar ganglia, plus an additional commissure that extends quite far posteriorly before reversing to extend anteriorly and subventrally to enter the preanal ganglion. Extending posteriorly from the lumbar ganglia are a pair of caudal nerves. A portion of each caudal nerve terminates in the ipsilateral member of the phasmids. The remaining portion of each caudal nerve continues past the phasmid to the extreme tail tip. In addition, fine ventrolateral and dorsolateral nerves extend anteriorly and posteriorly from each lumbar ganglion.
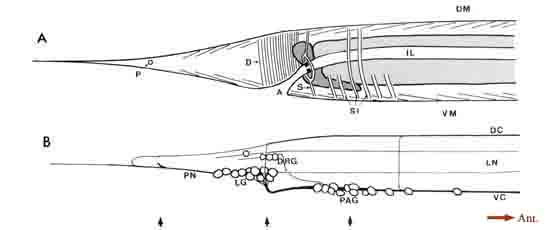 Figure 1. General anatomy of the tail. (A) Outline of the hermaphrodite tail viewed from the right side showing the tail musculature in relation
to the intestine, anus, and phasmidial opening. Intestinal cells are light gray; rectal gland cells are dark gray. Groups of muscle contractile units
are schematized as sets of thin parallel lines. (B) View of the nervous system from the right side (left lumbar ganglion, left lateral nerves, and leftward
commissures omitted). One pair of "lumbar" neuron somata lie separately, in register with the dorsolateral nerves. The right-hand member of this
pair is the unlabeled cell lying just posterior to the 3 DRG cells. Arrows at the bottom of the figure indicate the relative positions of thin sections
shown in Figure 3. For abbreviations, see Table 1.
Figure 1. General anatomy of the tail. (A) Outline of the hermaphrodite tail viewed from the right side showing the tail musculature in relation
to the intestine, anus, and phasmidial opening. Intestinal cells are light gray; rectal gland cells are dark gray. Groups of muscle contractile units
are schematized as sets of thin parallel lines. (B) View of the nervous system from the right side (left lumbar ganglion, left lateral nerves, and leftward
commissures omitted). One pair of "lumbar" neuron somata lie separately, in register with the dorsolateral nerves. The right-hand member of this
pair is the unlabeled cell lying just posterior to the 3 DRG cells. Arrows at the bottom of the figure indicate the relative positions of thin sections
shown in Figure 3. For abbreviations, see Table 1.
For purposes of reference in the following sections, Figure 2 shows reconstructions of several selected posterior neurons, viewed from the right side, and histograms showing the frequency of synaptic contacts within 3 regions of the tail. Figure 3 shows 3 of the coronal thin sections from which these reconstructions were made. Somata, axons, and dendrites of a limited number of neurons and muscle cells are marked, concentrating on features of the reconstructed cells from Figures 1A and 2. Figure 4 depicts relative cell body positions of all identified neurons from a dorsal aspect, using a symbolism denoting inferred function as well as position; representations are given for both B126 and B136 to show animal-to-animal reproducibility. Figures 5 and 6 show schematic reconstructions of every posterior neuron, viewed from a dorsal aspect. Figure 7 compares the positions of identified axons in both animals in the Iumbar-preanal commissures (Fig. 7A) and the preanal ganglion (8), in coronal sections.
Lumbar ganglion cells: anatomical description
and example of serial reconstruction of sensory neurons
Phasmidial neurons (PHA, PHB). The most obvious posterior sensilla are the 2 phasmids, which open laterally about 40 um posterior to the anus, at a point where the tail's diameter has diminished from about 70 um to about 15 um (Figs. 1, 2). Each phasmid consists of 2 ciliated dendritic endings, one ventral (PHA), and one dorsal (PHB), within an extracellular pocket formed by 2 accessory cells, the socket cell (Phsol) and the sheath cell (Phsh; Fig. 3A). The ciliated endings of PHA and PHB protrude through the sheath cell and appear to be in direct contact with the exterior of the animal. The phasmidial cilia have recognizable basal bodies but no striated rootlets. The ultrastructure of the phasmid has been shown in greater detail by Hall (1977) and by Sulston et al. (1980). These features suggest a chemosensory role for the phasmid and are homologous to those already described for 2 types of putatively chemosensory anterior sensilla, the amphids and the inner labial papillae (Ward et al., 1975; Ware et al., 1975).
Synaptic endings of PHA and PHB neurons occur only in the preanal ganglion; they contain large clear vesicles (50 ± 10 nm diameter), in contrast to the smaller vesicles (41 ± 9 nm diameter) seen in most other neurons. Figure 2A depicts the soma and processes of PHBR as an example, showing a lateral view. Figure 3 reveals 3 cross-sections from one animal in which PHBR can be seen as a ciliated ending (Fig. 3A), as a soma in the lumbar ganglion (Fig. 3B), and as an axon within the preanal ganglion (too small to be visible in the PAG area in Fig. 3C). The PHBR axon exits its soma and travels ventrally into a commissure within the section depicted in Figure 3B. Several thousand cross-sections were used to assemble detailed reconstructions of 2 animals at various magnifications to confirm this type of detail for every cell type. All of the neuronal and accessory cell somata positions are compared in Figure 4 for 2 animals. Figures 4 and 5 show schematic views from above, demonstrating the bilateral nature of the lumbar ganglia. Note that each PHB soma is located near the dorsal edge of the lumbar ganglion, near the exit of the lumbar-dorsal cord commissure. The positions of the PHB axons are easily seen in Figure 7, which depicts their location within the lumbar-preanal commissures (Fig. 7A) and within the preanal ganglion (8) in both B126 and B136. Note that each neuron is generally represented by only one profile in a given coronal section, as most neurons lack any secondary branching in the tail.
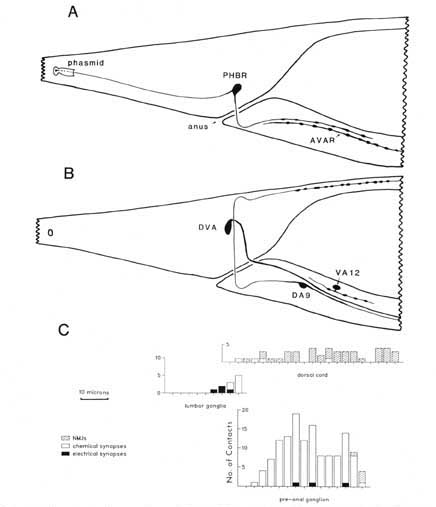 Figure 2. Serial reconstruction of neurons and synapse distribution. (A) Neurons PHBR and AVAR shown from the right side. This view was drawn approximately to scale by hand from serial reconstruction data. The approximate dimensions of the extracellular pocket of the phasmid is indicated. Swellings along the axon are not to scale; axon profiles are actually enlarged for clarity. (B) Neurons DA9, DVA, and VA12 shown schematically from the right, as above. C, Three histograms referenced to the above drawings illustrate the longitudinal distribution of synaptic contacts within the tail for B136, an animal in which every morphologically identifiable synapse has been cataloged. NMJs onto the defecation muscles are omitted, as they are not well demarked in B136. Numbers of synapses have been summed over intervals of 3 um. The general density of synaptic contacts in the ventral cord is much lower than in the preanal ganglion (White et al., 1976). For abbreviations, see Table 2.
Figure 2. Serial reconstruction of neurons and synapse distribution. (A) Neurons PHBR and AVAR shown from the right side. This view was drawn approximately to scale by hand from serial reconstruction data. The approximate dimensions of the extracellular pocket of the phasmid is indicated. Swellings along the axon are not to scale; axon profiles are actually enlarged for clarity. (B) Neurons DA9, DVA, and VA12 shown schematically from the right, as above. C, Three histograms referenced to the above drawings illustrate the longitudinal distribution of synaptic contacts within the tail for B136, an animal in which every morphologically identifiable synapse has been cataloged. NMJs onto the defecation muscles are omitted, as they are not well demarked in B136. Numbers of synapses have been summed over intervals of 3 um. The general density of synaptic contacts in the ventral cord is much lower than in the preanal ganglion (White et al., 1976). For abbreviations, see Table 2.
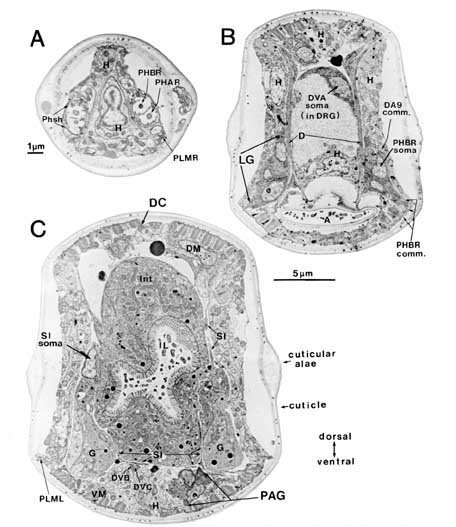 Figure 3. Sample sections from a reconstruction. Three coronal sections demonstrating some of the cell positions schematized in Figures 1 and 2 are shown. (A) Cut at the level of the phasmids. (B) Cut at the level of the lumbar and dorsorectal ganglia. (C) Cut at the level of the preanal ganglion. In (A) both pairs of chemosensory cilia can be seen within the extracellular pocket of the phasmid sheath cell. Arrows at the bottom of (B) indicate the relative positions of these 3 sections. Scale bars: (A) 1 um; (B) and (C) 5 um. For abbreviations, see Tables 1 and 2.
Figure 3. Sample sections from a reconstruction. Three coronal sections demonstrating some of the cell positions schematized in Figures 1 and 2 are shown. (A) Cut at the level of the phasmids. (B) Cut at the level of the lumbar and dorsorectal ganglia. (C) Cut at the level of the preanal ganglion. In (A) both pairs of chemosensory cilia can be seen within the extracellular pocket of the phasmid sheath cell. Arrows at the bottom of (B) indicate the relative positions of these 3 sections. Scale bars: (A) 1 um; (B) and (C) 5 um. For abbreviations, see Tables 1 and 2.
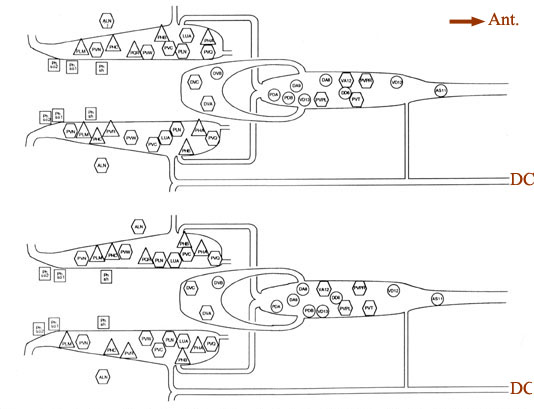 Figure 4. Cell body positions. Schematic views in 2 animals (B126, above; B136, below) of locations of cell bodies of neurons and accessory cells. The view is from a dorsal aspect, with the most dorsal region peeled away to the bottom of each panel. Cell bodies of ALN neurons lie in dorsal positions outside the lumbar ganglia proper. Labels for each soma are placed inside symbols indicating presumed functions; actual soma shapes are generally larger and more space filling. Triangles. sensory neurons; hexagons, interneurons; circles. motoneurons; squares. accessory cells of the phasmids. For abbreviations, see Table 2.
Figure 4. Cell body positions. Schematic views in 2 animals (B126, above; B136, below) of locations of cell bodies of neurons and accessory cells. The view is from a dorsal aspect, with the most dorsal region peeled away to the bottom of each panel. Cell bodies of ALN neurons lie in dorsal positions outside the lumbar ganglia proper. Labels for each soma are placed inside symbols indicating presumed functions; actual soma shapes are generally larger and more space filling. Triangles. sensory neurons; hexagons, interneurons; circles. motoneurons; squares. accessory cells of the phasmids. For abbreviations, see Table 2.
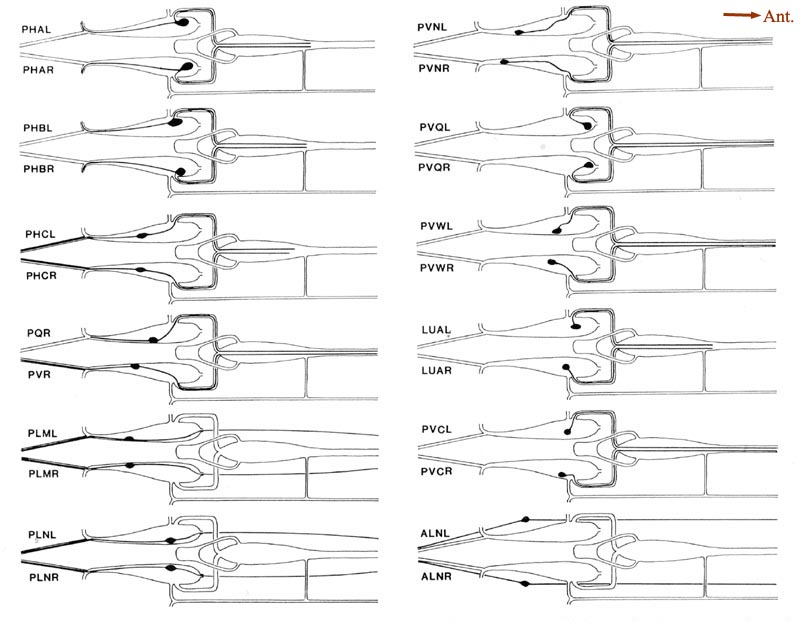 Figure 5. Schematic models of all lumbar ganglion neurons. The arborization of each lumbar neuron is shown, using the same perspective as in Figure 4. Because these cells have no secondary branches, the schematic model is a fair representation of the actual cell shape. The caliber of the neurites has been enlarged for clarity. No attempt is made to show the close approximations of homologous neurites as they traverse the preanal ganglion. The processes of PLML/R and PLNL/R travel in ventrolateral positions, and those of ALNL/R travel in dorsolateral positions. For abbreviations, see Table 2.
Figure 5. Schematic models of all lumbar ganglion neurons. The arborization of each lumbar neuron is shown, using the same perspective as in Figure 4. Because these cells have no secondary branches, the schematic model is a fair representation of the actual cell shape. The caliber of the neurites has been enlarged for clarity. No attempt is made to show the close approximations of homologous neurites as they traverse the preanal ganglion. The processes of PLML/R and PLNL/R travel in ventrolateral positions, and those of ALNL/R travel in dorsolateral positions. For abbreviations, see Table 2.
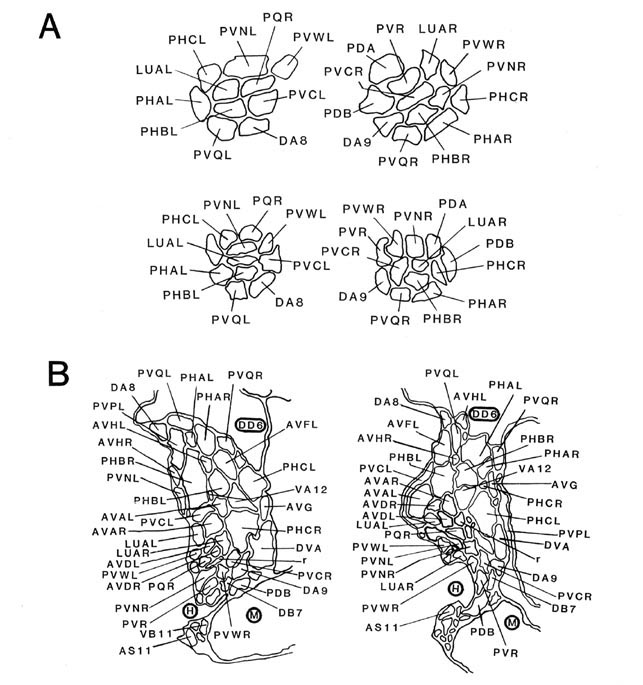 Figure 7. Coronal sections of the ventrolateral commissures and the preanal ganglion. A, Left and right ventrolateral commissures are shown for B126 (top) and B136 (bottom), as tracings from equivalent regions in the 2 animals. Besides the lumbar neurites, which predominate, there are also 3 neurites from preanal ganglion neurons that are passing toward the dorsal cord via these commissures. B, Sections from the anterior portion of the preanal ganglion in B136 (left) and B126 (right). Because neurons are largely unbranched, most are represented as single profiles. Note that bilateral homologues generally lie quite close to one another. B126 has been rotated 30° clockwise for convenience in presentation. The soma of DD6 is marked by a heavy circle, as are neighboring muscle (M) and hypodermal (H) cells. Magnification is approximately 25,000x. For abbreviations, see Table 2.
Figure 7. Coronal sections of the ventrolateral commissures and the preanal ganglion. A, Left and right ventrolateral commissures are shown for B126 (top) and B136 (bottom), as tracings from equivalent regions in the 2 animals. Besides the lumbar neurites, which predominate, there are also 3 neurites from preanal ganglion neurons that are passing toward the dorsal cord via these commissures. B, Sections from the anterior portion of the preanal ganglion in B136 (left) and B126 (right). Because neurons are largely unbranched, most are represented as single profiles. Note that bilateral homologues generally lie quite close to one another. B126 has been rotated 30° clockwise for convenience in presentation. The soma of DD6 is marked by a heavy circle, as are neighboring muscle (M) and hypodermal (H) cells. Magnification is approximately 25,000x. For abbreviations, see Table 2.
Neuron wilh a buried ending (PQR). A third type of accessory cell, the wing cell (Phso2), is more loosely associated with each phasmid, sending abroad, winglike process along much of the phasmid's medial surface. Near the base of the left phasmid, but not the right, the process of Phso2 is invaginated by a different type of ciliated ending, PQR, for which it provides a thin wrapping for some distance posteriorly (Hall, 1977; Sulston et al., 1980). The basal body of the PQR cilium is located near the point of entry into the wing cell, and the distal portion of the ending is markedly spatulate. From its location near the axis of the tail, the PQR ending might be preferentially sensitive to pronounced, deep mechanical deformations.
Neurons with postphasmidial processes (PHC). Posterior to the phasmids, each caudal nerve contains a small process (PHC) that runs in a ventrolateral position for 65 um to the extreme tip of the tail. As the tail tip narrows posteriorly, the 2 processes come to lie adjacent to one another, and for a considerable distance, the fine tail tip consists only of these 2 processes, surrounded by a thin hypodermal layer and the overlying cuticle (Hall, 1977). This fine tip undergoes pronounced deformation, often folding back on itself, when the animal moves backwards. Although the PHC processes are not ciliated, their physical location suggests a possible mechanosensory function. Like the PHA and PHB sensory neurons, the PHC neurons have distinctively larger vesicles at their synapses than do other posterior neurons.
Neurons with microtubule-filled processes (PLM. PVR). From each lumbar ganglion there extends, both anteriorly and posteriorly, a fine ventrolateral nerve consisting of 2 processes. As has been previously described (Chalfie and Thomson, 1979), 1 of these 2 processes (PLM) contains a distinctive array of about 40 microtubules. Each PLM process runs quite near the surface, separated from the cuticle by only a thin layer of hypodermal cytoplasm. The PLM processes are sensory receptors for "light touch" delivered to the posterior portions of the animal (Chalfie and Sulston, 1981; Chalfie et al., 1985).
One unpaired lumbar neuron, PVR, sometimes has a posterior process running caudally from its soma to a dorsal position in the extreme tail tip (White et al., 1986). In B126, this process contains a small bundle of microtubules, suggesting a sensory function. In B136, no posterior process is found for PVR. Immunochemical staining has revealed that the microtubule bundle in PVR shares the same acetylated alpha-tubulin that is otherwise characteristic of 3 pairs of mechanosensory cells (PLM, AVM, ALM; Siddiqui et al., 1989).
Dorsorectal ganglion cells
DVA, DVB, and DVC each send single processes rostrally through circumrectal commissures into the preanal ganglion and thence into the ventral nerve cord (Figs. 2B and 3B show DVA as an example). The DVB process is greatly enlarged and filled with synaptic vesicles as it crosses the ventral hypodermal ridge to enter the preanal ganglion. Although postsynaptic specializations could not be seen, it seems likely that DVB acts as a defecation motoneuron with synaptic outputs onto arms of the defecation muscles (D, S, SI, VM), which lie along the dorsal surface of the hypodermal ridge. The proximity of the DVB axon and the SI muscle arms can be noted in Figure 3C.
Preanal ganglion cells
Interneurons. Of the 13 neurons with cell bodies in the preanal ganglion, only VA12 has significant synaptic output to neurons within the preanal ganglion itself. VA12 also receives a number of synaptic inputs in the ganglion and thus qualifies as an interneuron. However, VA12 additionally sends a process forward into the ventral nerve cord, which makes a number of NMJs of the type previously described as "class A " (White et al., 1976, 1986). Thus, VA12 acts both as an interneuron and as a motoneuron. PVPL/R and PVT are also intemeurons; most of their synapses are in the ventral cord or the nerve ring (White et al., 1986).
Motoneurons. Eight of the 13 neurons of the preanal ganglion are motoneurons, but because most NMJs to ventral body muscles occur anterior to the preanal ganglion, few of these junctions were seen in our reconstructed series. A reconstruction of the dorsal cord in B136 revealed many NMJs for some of these neurons.
Two neurons, DA8 and DA9, send anterior processes into both the dorsal and the ventral nerve cords (Fig. 2B shows DA9 as an example). These are "class A " motoneurons (White et al., 1976), and in both the dorsal and the ventral nerve cords, their processes run together in the position typical of class A processes. In the dorsal cord, DA9 was found to make a series of NMJs in the reconstructed region of B136. In the same region, DA8 had no NMJs, but its dorsal process extended anteriorly beyond the region of reconstruction. Its expected territory for NMJs should lie just rostral to that of DA9 (White et al., 1976). VA12 is also a class A motoneuron, with NMJs along the ventral cord. VA11 lies rostral to the preanal ganglion proper, outside the region reconstructed, but is listed as part of this set of motoneurons due to its lineal relationship to other preanal neurons (cf. Sulston and Horvitz, 1977). PDA forms NMJs along the dorsal cord.
Three neurons, VD13, VD12, and DD6, send single processes forward from the preanal ganglion into the ventral cord. Each process has a commissure more anteriorly, which goes to the dorsal cord (White et al., 1986), and in the dorsal cord, we have identified what appear to be the caudal projections from these commissures. VD13 and VD12 receive synaptic input in the dorsal cord and make NMJs in the ventral cord, whereas DD6 receives input ventrally and makes NMJs dorsally. These neurons are thus typical "class D" motoneurons (White et al., 1976), and in the portion of the dorsal cord we have reconstructed, their processes run in the positions typical of class D processes (VDz, VDy, DDz in Table 3).
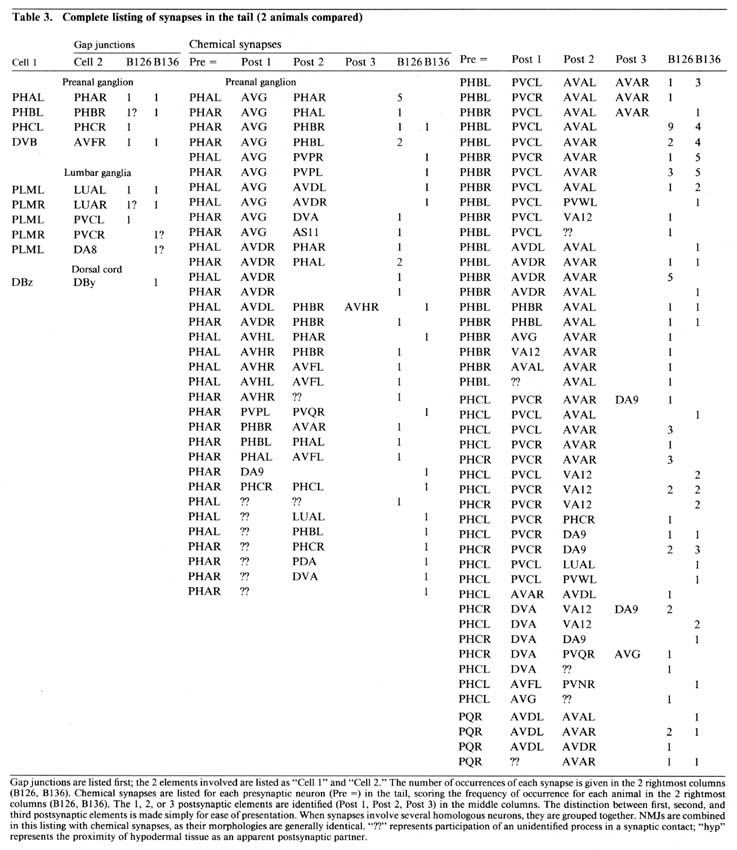 Table 3. Gap junctions are listed first; the 2 elements involved are listed as "Cell 1" and "Cell 2." The number of occurences of each synapse is given in the 2 rightmost columns (B126, B136). Chemical synapses are listed for each presynaptic neuron (Pre =) in the tail, scoring the frequency of occurence for each animal in the 2 rightmost columns (B126, B136). The 1, 2, or 3 postsynaptic elements are identified (Post 1. Post 2, Post 3) in the middle columns. The distinction between first, second, and third postsynaptic elements is made simply for ease of presentation. When synapses involve several homologous neurons, they are grouped together. NMJs are combined in this listing with chemical synapses, as their morphologies are generally identical. "??" represents participation of an unidentified process in a synaptic contact; "hyp" represents the proximity of hypodermal tissue as an apparent postsynaptic partner.
Table 3. Gap junctions are listed first; the 2 elements involved are listed as "Cell 1" and "Cell 2." The number of occurences of each synapse is given in the 2 rightmost columns (B126, B136). Chemical synapses are listed for each presynaptic neuron (Pre =) in the tail, scoring the frequency of occurence for each animal in the 2 rightmost columns (B126, B136). The 1, 2, or 3 postsynaptic elements are identified (Post 1. Post 2, Post 3) in the middle columns. The distinction between first, second, and third postsynaptic elements is made simply for ease of presentation. When synapses involve several homologous neurons, they are grouped together. NMJs are combined in this listing with chemical synapses, as their morphologies are generally identical. "??" represents participation of an unidentified process in a synaptic contact; "hyp" represents the proximity of hypodermal tissue as an apparent postsynaptic partner.
Neurons of uncertain function. AS11 and PDB would be expected from their lineages (fourth-most anterior daughters of P neuroblasts) to be "class A " motoneurons with a short dorsal process forming NMJs (Sulston, 1976; Sulston and Horvitz, 1977). AS11 differs from other AS motoneurons, which have only a rostral branch in the dorsal cord. AS11 also lacks any NMJs along its dorsal processes (followed to their endpoints in B136). However, both the dorsal and ventral processes of AS11 run in positions typical of other AS motoneurons, and the synaptic input to AS11 in the ventral cord resembles input to other class A motoneurons. Thus, in many respects, AS11 resembles atypical AS motoneuron, but in the absence of observed output, its function remains unclear.
The posterior process of PDB travels by an unusual ventral route far into the tail tip (more than 10 um posterior to the phasmidial openings) before coursing dorsally and returning anteriorly to enter the emerging dorsal cord (Figs. 1B, 6). The dorsal process travels anteriorly as part of the dorsal nerve cord and terminates within the region we have reconstructed in B136. Neither the ventral processes nor the dorsal process of this cell have synaptic connections that we can observe. A few NMJs may occur in the dorsal cord according to White et al. (1986). The deviation of AS11 and PDB from their expected fates resembles similar deviations observed among the daughters of extremely anterior P neuroblasts (Sulston and Horvitz, 1977). There remains the possibility that PDB, AS11, and other tail neurons might also be involved in synapses that remain morphologically undetected by present methods. Small gap junctions, in particular, are often difficult to identify in conventional thin sections (cf. Hall et al., 1983). They might also be involved in transient synaptic interactions at another developmental stage.
Neurons with anterior somata
A number of anterior neurons, whose somata lie rostral to the region reconstructed, send axons into the preanal ganglion via the ventral cord. Processes of 13 neurons have sufficiently distinctive characteristics within the preanal ganglion itself to permit repeated identification in different individuals, and we were able, with varying degrees of certainty, to associate all but one of these with specific identified anterior somata by the following criteria: In the limited portion of the ventral nerve cord we reconstructed anterior to the preanal ganglion, the general structure of the cord is very similar to that described previously for the anterior ventral cord (White et al., 1976). Accordingly, characteristic features such as fiber size, cytoplasmic appearance, and relative position within the cord were used to identify 3 major pairs of cord interneurons (PVC, AVA, AVD), 2 of which had anterior somata (AVA, AVD; Hall, 1977). The patterns of synaptic interaction involving major interneurons in the tail are largely as predicted by homology with the anterior regions of the ventral cord (White et al., 1976, 1986; Hall, 1977). The caudal extension of AVAR into the preanal ganglion is depicted in Figure 2A as an example. In the same fashion, several motoneuron axons could be identified by their relative positions in the caudal region of the motor end plates.
Our results were compared by White with a series that extends further anterior (White et al., 1986); this comparison confirmed all of our major interneuron identifications. Based on fiber positions and in some cases on limited amounts of specific synaptic input, White could also provide a more tentative identification of 7 other interneuron processes, leaving only 1 process unidentified (J. G. White, personal communication). Figure 6 shows schematic views of ALA, AVHL, and AVFR axons.
A few of these anterior processes have unique features that are not shown in the figures. The 2 AVH processes run near the dorsal extreme of the ganglion and are distinguished by their relatively small size compared to their neighbors. Another pair, AVFL/R, also run near the dorsal extreme of the ganglion but are larger and have a series of rather marked swellings along their lengths. The AVFR process continues caudally through the preanal ganglion, enters the right circumrectal commissure, then passes over the anal ridge where its swellings occur. AVFL ends in swellings within the preanal ganglion. The AVF processes also have a series of vesicle-filled swellings in the head (White et al., 1986). AVG runs as a flattened profile on the right of the preanal ganglion and usually enters the right circumrectal commissure together with DVA and AVFR. (This feature was not observed in an animal reconstructed by Lois Edgar). AVL was identified in only 1 of 2 animals (B126), where it enters the left circumrectal commissure with DVB and DVC. An unidentified process (Fig. 7B, r) runs near the middle of the preanal ganglion in both B126 and B136; it has few synaptic interactions and no other obvious distinguishing features.
Single processes running in the 2 lateral hypodermal cords are probably the distal extensions of the ALA neuron, a single cell whose soma lies in the dorsal ganglion in the head. These 2 processes terminate by invaginating into the PVC somata to form a series of synapses. Identification of these processes is still tentative (White et al., 1986; White, personal communication).
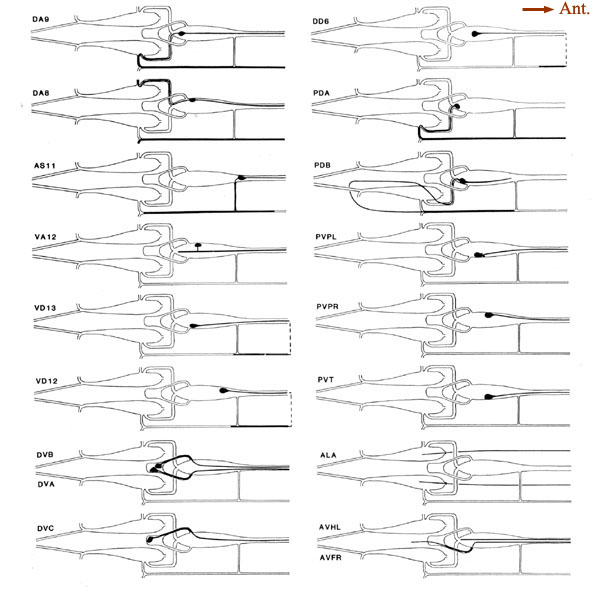 Figure 6. Schematic models of all preanal and dorsorectal ganglion neurons. Arborizations are shown using the same conventions as in Figure 5. PVPL is shown with 2 short extra branches. Other preanal neurons lack such extra branches. The route of the PDB commissure is unusual. The lower 2 panels on the right show the posterior arbors of a few neurons with anterior somata. The 2 axons from anterior neuron ALA lie in the lateral nerves. Broken line. indicate commissures that lie outside the reconstructed region. For abbreviations, see Table 2.
Figure 6. Schematic models of all preanal and dorsorectal ganglion neurons. Arborizations are shown using the same conventions as in Figure 5. PVPL is shown with 2 short extra branches. Other preanal neurons lack such extra branches. The route of the PDB commissure is unusual. The lower 2 panels on the right show the posterior arbors of a few neurons with anterior somata. The 2 axons from anterior neuron ALA lie in the lateral nerves. Broken line. indicate commissures that lie outside the reconstructed region. For abbreviations, see Table 2.
Ordering of processes within bundles
There are 5 regions of the tail in which many neuronal fibers course together as a bundle through surrounding tissue; these are the 2 ventrolateral commissures (left and right), the preanal ganglion itself (which is in essence a bundle of parallel fibers), and the posterior dorsal and ventral cords. Within such bundles, a degree of ordering is observed that appears as reproducible as the positioning of cell bodies. Figure 7A illustrates this ordering at 1 level of the left and right ventrolateral commissures, for both B126 and B136; the ordering is most easily appreciated by comparing small groups of fibers (e.g., PHA, PHB, and PVQ) with their bilateral homologues across the midline or with their direct homologues in the second animal. While the ordering is certainly not absolute, it is clearly nonrandom. Within these bundles, there is a loose relationship between cell body position and fiber position; lumbar neurons with the most rostral cell bodies tend to have fibers that lie on the ventral side of the commissure.
Within the preanal ganglion itself, as shown in Figure 7B, the number of fibers is larger, but a similar sort of ordering persists. Interestingly, homologous fibers from the 2 lumbar ganglia come to lie close to one another in the preanal ganglion, suggesting some sort of homologue affinity or a common response to guiding principles (cf. White et al., 1983). In addition, the ordering is dorsoventrally inverted relative to the ventrolateral commissures; those fibers running most ventrally in the commissures occupy dorsal positions in the preanal ganglion.
General distribution and appearance of synapses
Table 3 lists all the synaptic interactions observed in the 2 animals examined. Chemical synapses are the predominant class. Multiple separate punctate contacts involving the same cells are common; each such contact is counted separately. Synaptic interactions involving unidentified processes number less than 10% of the total interactions. NMJs are also listed, including those in the dorsal cord.
The few synapses outside the preanal ganglion occur in the dorsal cord or within the lumbar ganglia or their associated commissures. In the lumbar ganglion, reproducible chemical synapses are formed by a pair of lateral fibers (perhaps ALA) to the PVC interneurons. These synapses are monadic and occur in specialized depressions invaginating the surface of the PVC somata. Reproducible gap junctions in the ventrolateral commissures occur between processes of the PLM sensory neurons and the LUA interneurons. Nonreproducible (i.e., single-instance) lumbar gap junctions were also observed between PLM processes and several other cells (Table 3, gap junctions), suggesting a particularly strong tendency of PLM cells to form gap junctions within the lumbar ganglia.
Within the preanal ganglion, few gap junctions were observed reproducibly (i.e., in both animals). Many of the cases listed in Table 3 involved a bilaterally homologous sensory cell pair. In the animal examined by White et al. (1986), a somewhat larger number of gap junctions were identified, including most of those described here. Of the additional gap junctions they noted, many are between other bilateral homologues. Others are between nonhomologues that are also involved in chemical synapses with each other, as well as some additional PLM contacts in the ventrolateral commissures.
Reproducibility of interactions. In the listings in Table 3, bilateral homologues are clearly involved in similar patterns of chemical synaptic interaction. All contacts involving either the right or left member of each cell pair have been grouped together in order to emphasize this point. The number of synapses seen between most 3-cell groups are not highly repeated in a given animal or when comparing B126 to B136. In fact, many specific dyadic interactions are seen only once in one animal and not at all in the other. However, when one sums all of the contacts between cell pairs disregarding dyadic partners the correspondence is much better (Table 4; Hall, 1977), and when one combines the data from bilateral homologues, the correspondence is very high (Table 5; see Discussion).
Each neuron synapses onto a restricted set of cells. For most presynaptic neurons, the list of postsynaptic targets is rather small, and certain pairs of postsynaptic targets are clearly preferred (see Discussion). A few presynaptic neurons do not appear to focus on preferred pairs, but choose a wider assortment of targets (e.g., PHA and DVB) without many repeated contacts to the same targets. PHB synaptic outputs are more typical. Note, in Table 3, the number of different permutations by which PHBL/R neurons contact PVCL/R and AVAL/R simultaneously, and the relatively large number of these contacts in each animal. There is a preponderance of ipsilateral contacts among the PHB->PVC+AVA synapses, and PVCL receives many more contacts than PVCR. This ipsilateral bias seems to stem mostly from an incomplete mixing of left and right lumbar homologues as they enter the preanal neuropil; more rostral portions of the preanal neuropil show more frequent contralateral contacts in many synapse classes.
A few neurons are contacted only infrequently by PHB neurons. By comparing data from several animals, it is possible to determine that the less common synaptic targets are, in fact, quite variable and may be contacted once or twice in some animals, but not in others. This fact is more clearly noted by ignoring dyadic relationships, and reclassifying all synaptic contacts onto a 2-way table. Table 4 shows a subset of our data from Table 3, displaying the number of synaptic contacts between 26 neurons in the tail, where a dyadic synapse made by PHBL-> PVCL + VA12 is shown as 2 contacts: one from PHBL-> PVCL and another from PHBL-> VA12. For ease of presentation, Table 4 shows only about half of the possible synapse combinations, which require a 40 x 40 matrix to include all cells present in the preanal neuropil. However, Table 4 is arranged to include almost all common synapse types; most of the other boxes in the full 40 x 40 matrix are empty. Table 4 also includes the same type of data obtained from an animal reconstructed by White et al. (1986), using numbers compiled from their illustrations. Some synapses are very highly conserved, especially PHBL-> PVCL, which is the most highly repeated contact in each animal. As discussed below, we designate synapses that are repeated 6 times or more in the 3 animals combined as the normal targets (lumping all contacts involving bilateral homologues or homologous motoneurons). Thus, the PHB-> PVC boxes are marked by a heavy outline, as are the less common PHB-> PHB boxes. The latter are exclusively contralateral as there are no self-synapses, whereas the former are more often ipsilateral. There are scattered synaptic contacts shown in Table 4 that do not fall within the heavily outlined boxes; these are considered "infrequent synapses," and below, we discuss the possibility that they represent developmental "noise". When the entire data set is examined on a 2-way table, the number of infrequent synapses may total about 20% of all contacts, depending on how they are defined.

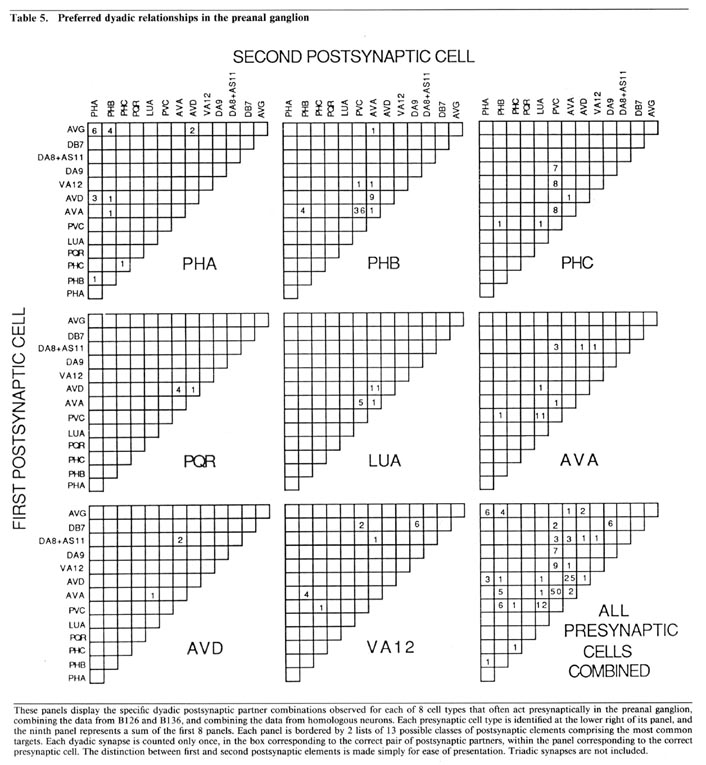
Partner relationships in dyadic synapses. The majority of the dyadic chemical synapses in the preanal ganglion occur between a subset of 21 neurons. The data in Table 5 is derived from that in Table 3 by combining the results from B126 and B136 and by combining the interactions of bilateral homologues. Table 5 lists the possible pairs of postsynaptic partners in a set of 2-dimensional arrays, one array for each important presynaptic neuron class. In each case, the dyadic contacts for a class of presynaptic neurons are listed singly, each position in the array corresponding to the 2 possible types of postsynaptic neurons involved (i.e., as combinatorial pairs of postsynaptic partners).
Each array in Table 5 includes, as its diagonal, positions for cases in which the 2 postsynaptic partners are homologues. Examination of Table 5 reveals that these diagonals are virtually empty, indicating that bilateral homologues are contacted together very rarely. This is true despite the fact, as shown in Table 3, that it is extremely common for a given presynaptic neuron to contact both members of a bilaterally homologous pair, overall. This can occur, of course, only if the 2 bilaterally homologous cells are virtually always contacted separately, in different punctate contacts, as is indeed the case. This observation suggests that there is a proscription against bilateral homologue involvement at the single contact level, but that this proscription does not apply globally when contacts are summed.
While the array diagonals are surprisingly empty, each array is characterized by the grouping of observed contacts into a few specific positions, each representing the joint occurrences of a postsynaptic neuron of one specific type with another of a different specific type. The importance of these groupings is supported by examination of the minority of triadic synapses (cf. Table 3); about 75% of these can be considered as modified versions of one or another of the common dyadic classes, in the sense that the first 2 postsynaptic neurons are as in a common dyadic class, and the third is a bilateral homologue of 1 of the first 2. We believe, as discussed below, that these dyadic (and triadic) groupings are functionally important for the divergence of information into a limited number of alternative routes for processing.
Wiring diagram. As the foregoing section makes clear, in the desired wiring diagram, the predominant synapses can be accurately represented as double-headed arrows, going from one presynaptic cell type to 2 different postsynaptic ones. In Figure 9, this symbolism is used, and the thickness of the arrows is used to indicate the frequency of the corresponding synapse type, as judged by the number of separate punctate contacts (though true physiological synapse strength obviously cannot be inferred from this data).
Certain features, probably of functional importance, stand out in the wiring diagram of Figure 9. First, it is striking how predominant is one class of synapses, that from the PHB sensory cells onto the 2 major interneuron types AVA and PVC. It is noteworthy that the interaction pattern of the PHB cells is quite different from that of the PHA cells, even though these 2 types of sensory cells have adjacent ciliated dendrites in the phasmidial pocket. Both phasmidial neuron types PHA and PHB exhibit what appear in the representation to be self-synapses, but are in fact synapses from one bilateral homologue to the other. There is a strong tendency towards convergence of several sensory neuron outputs onto 3 major interneuron classes, AVA, PVC and AVD; these are major interneurons of the ventral cord and the nerve ring. Frequently, the 2 postsynaptic cells types jointly contacted in a given type of synapse (e.g., AVA and PVC) make direct contact with one another in other synapses; interestingly, such contacts are usually unidirectional (e.g., AVA is presynaptic to PVC but not vice versa). There are few instances of reciprocal synapse formation; in our data, they only involve the major interneuron AVA and the loeal interneuron LUA. [The data in White et al. (1986) include a few reciprocal contacts between AVA and PVC and between LUA and AVD.] Interestingly, and as a corollary of the previous point, there are few dyadic synapses in which AVA and LUA are jointly contacted (none in our data, 4 instances in White's data); indeed, the only significant dyadic input to LUA is from AVA. Finally, for each presynaptic cell type having several different outputs, there is a very strong tendency for the several outputs to share one postsynaptic partner in common, usually either AVA or PVC.
In the discussion, these features serve as the focus of an attempt to understand both the function and the development of this synaptic circuitry.
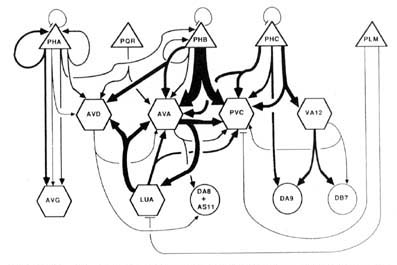
Figure 9. Wiring diagram for the tail. Double-headed arrows indicate dyadic chemical synapses. The width of individual lines is proportional to the relative frequency of occurrence of synaptic contacts. Excluded from the diagram, but probably of functional significance, are the following frequent chemical synapse classes: PHA->AVH, PHC->DVA, ALA->PVC, and DVB->DVC. Many of the interneurons have many additional synaptic interactions either in the ventral nerve cord or in the nerve ring. Triangles, sensory neurons; hexagons, interneurons; circles, motoneurons; -|, gap junctions. See Results, Wiring diagram, for format; see Table 2 for abbreviations.
Discussion
Identity and function of posterior neurons
Cell identification and reproducibility Each of the 40 neurons of the C. elegans posterior nervous system has a reproducible set of features by which it can be unambiguously identified in different individuals. These features include cell body position, number and direction of fiber projections, and size and cytoplasmic appearance of the fibers. For each of the different sorts of features, the degree of reproducibility between isogenic individuals appears to be as good as that bilaterally within a given individual. Table 2 provides a summary list of some of the most salient features and a correspondence of naming systems between previous publications.
There is a remarkable economy of cell number, cell shape, and synaptic arrangements. The number of neurons serving any particular function is very small. Neurons with combined functions occur, and there are few layers of interneurons interposed between sensory cells and motor cells. Each neuron lacks secondary branches within the tail region; the anterior nervous system is fairly similar in this respect. The absolute number of synaptic contacts per neuron or per synapse class is certainly not large, even compared to most invertebrates. This limited set of reproducible neurons with distinct functional roles offers unique opportunities for physical manipulation of the nervous system, by laser ablation or by genetic or pharmacological means.
Cell function: sensory reception and processing. A principal function of the C. elegans posterior nervous system appears to be the reception and processing of information from posterior sensory receptors. Previous behavioral and genetic studies have shown that C. elegans is able to distinguish chemical stimuli (Ward, 1973; Dusenbery et al., 1975), mechanical stimuli (Chalfie and Sulston, 1981), optical stimuli (Burr, 1985), osmotic gradients (Culotti and Russell, 1978), and thermal gradients
(Hedgecock and Russell, 1975). Morphological studies of sensory mutants indicate that many of the above capabilities derive from the amphidial sense organs (Lewis and Hodgkin, 1977; Albert et al., 1981; Hedgecock et al., 1985; Perkins et al., 1986). Response to light touch is localized to 6 neurons whose microtubule-fllled mechanocilia lie along the lateral margins of the head, body, and tail (Chalfie et al., 1985). No sensory mutant has yet shown morphological changes restricted to the phasmids, but several chemosensory and osmotic avoidance mutants show similar defects in both amphids and phasmids (Perkins et al., 1986).
The phasmids appear likely on anatomical grounds to be chemosensory. The changes in phasmidial staining or in phasmidial cilia ultrastructure noted in several chemosensory mutations support this conclusion (Perkins et al., 1986). Their extreme posterior placement suggests comparison of phasmid signals with signals to the anterior amphids. Because the phasmids could be involved in detecting both attractants and repellents, and because each contains 2 ciliated neurons (PHA, PHB), we speculate that the synapses of one neuron class might promote forward movement; the other, backward movement. Indeed, the synaptic output of PHA and PHB are strikingly different, as discussed below.
Bilaterally homologous sensory cells are probably functionally identical. There are 4 pairs of bilaterally homologous sensory cells (PLM, PHA, PHB, PHC), which are very nearly mirror images of one another at all anatomical levels from gross cell position down to their axon and dendritic projections. There remains the question whether 2 sensory homologues act in concert with one another or in a lateralized manner, perhaps for purposes of orientation. Lateralization seems unlikely for many reasons. The paired sensilla for PHA, PHB, and PHC are arranged so close to one another and in such an orientation as to effectively preclude differential stimulation of one member of the pair. Chalfie and Sulston (1981) have reported that laser ablation of a single member of the PLM pair "does not detectably affect touch sensitivity." Also, the 2 homologous members of a sensory pair often form gap junctions and/or chemical synapses onto one another. The gap junctions, in particular, are difficult to reconcile with the notion of lateralized function. Finally, the synaptic outputs from the homologous members of a sensory pair, rather than occurring to separate neurons for potentially separate parallel processing, are instead largely overlapping. This feature is not evident in Figure 9, where homologues are combined, but can be seen in Tables 3 and 4, where they are kept separate. As noted above, the excess of ipsilateral contacts by some sense cells seems to reflect incomplete mixing of left and right lumbar processes near the posterior limit of the preanal ganglion.
Sensory information from the tail is primarily converged onto a few major interneurons. The output of most tail receptors converges onto AVA, AVD, and PVC interneurons (Fig. 9). A model of the ventral-cord circuitry based upon laser ablations of motoneurons or interneurons (Chalfie et al., 1985; Chalfie and White, 1988) suggests that PVC and AVB interneurons can stimulate class B motoneurons to promote forward locomotion, while AVA and AVD interneurons can stimulate class A motoneurons to promote backwards locomotion. This model for forward versus backward motion is reasonably consistent with neurophysiological and biochemical studies of identified motoneurons in a larger nematode species, Ascaris (Stretton et al., 1985). We speculate that the very common PHB->AVA, PVC synapses may underlie an escape response. If the PHB cells were sensitive to chemical repellents, the net effect of PHB excitation of PVC would be to promote rapid forward movement and hence escape. The simultaneous PHB contacts onto AVA might be inhibitory, shutting down backwards motion, thus sharpening the escape response. Similarly, stimulation of the PHC mechanoreceptors could also excite PVC and result in forward movement. The gap junctions found in the posterior ventral nerve cord between the PLM touch neurons and PVC interneurons (Chalfie et al., 1985; White et al., 1986) are consistent with this general notion. These speculations may be testable by selective laser ablations or perhaps by genetic dissection of the circuits.
The PHA chemoreceptors are well situated to allow comparisons between amphid and phasmid. PHA sense cells synapse primarily onto other sense cells and onto the AVG interneuron. The sensory feedback of PHA onto other tail receptors might serve to inhibit other sensory modalities during PHA activity. From the wiring diagrams of White et al. (1986), the following circuit is evident:
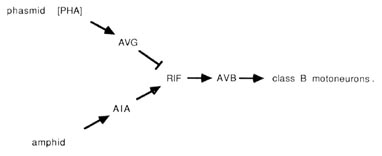
The PHA outputs onto AVG can potentially conduct a phasmidial signal to the head, where AVG synapses via large gap junctions onto the RIF interneurons. In the nerve ring, RIF interneurons also receive processed sensory signals from amphidial chemoreceptors via the AIA interneurons. Thus, RIF interneurons may compare processed chemosensory signals from head and tail. In turn, the RIF interneurons have prominent synaptic inputs onto the AVB interneurons, which may reset the animals body motion in response to the compared chemical stimuli. As noted above, the AVB interneurons are prominent components of the ventral cord wiring, contacting class B motoneurons along the length of the body and thus controlling forward motion.
Function and development of dyadic synapses
The dyadic synapse provides a basic unit for processing. One of the most striking features of the preanal ganglion circuitry is the preponderance of dyadic synapses. The 2 postsynaptic partners in a given contact are almost never homologous (Table 5); this makes sense if the function of the dyadic synapse is to diverge the information into distinct channels with different opportunities for modification. We presume that the 2 postsynaptic partners are affected simultaneously and proportionately by the presynaptic neuron. In many cases, the 2 postsynaptic cell types make direct contact with one another elsewhere, and most such contacts are in a single direction (Fig. 9). This circumstance can be represented diagrammatically as follows:
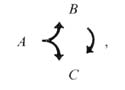
where A is the original presynaptic cell, and B and C are the 2 original postsynaptic partners. In this circumstance ("feed-forward"), cell C may receive 2 versions of an initial synaptic output from cell A; the first is direct, via the original dyadic synapse, and the second is indirect, and potentially modified, through cell B. The indirect version, of course, could depend on the state of cell B; it might arrive with a time delay, with either an enhancing or an opposing effect, with an altered duration, and so forth. This basic 3-cell configuration could serve a variety of functions, for example, prolongation of the signal's effets, gating the signal's effects with respect to a threshold in cell C or selecting for signals with a pronounced rate of change.
Dyadic and triadic synapses are well known anatomically in many other sensory ganglia, both in vertebrates and in invertebrates, but have not been widely studied physiologically. While their function is not yet well understood, current information seems compatible with the divergence/reconvergence idea presented above. Specific combinations of postsynaptic partners are also known to be preferred at tetradic photoreceptor terminals in the fly's visual system (Nichol and Meinertzhagen, 1982; Fröhlich, 1987), and feedback relationships of these synapses appear to be functionally plastic (Kral and Meinertzhagen, 1989). Dyads are involved in feedback relationships in the dragonfly ocellar retina (Klingman and Chappell, 1978) and in both feed-forward and feedback relationships in the visual system of the desert ant (Meyer, 1979). Physiological studies of vertebrate dyadic synapses have explored their feedback relationships (cf. Byzov and Golubtsov, 1978; Raviola and Dacheux, 1987). (In the vertebrate literature, dyadic synapses are often called "triads," and triadic synapses may be called "tetrads." In other instances, "triads" may refer to serial synapses.)
Infrequent synapse classes. There are many synapses listed in Table 3 that do not fall into one of the common synapse classes; that is to say, they involve a postsynaptic partner that is rarely contacted at all, or rarely by the particular presynaptic neuron. Because practically all of the individual synapse categories involve relatively low numbers on an absolute scale, it is difficult to define an infrequent synapse class on a simple numerical criterion. Depending upon what criterion is selected, infrequent synapses may represent 10-25% of all contacts.
Within the synaptically active cell group listed in Table 5, infrequent synapse classes accounted for only 28 of the 427 contacts observed in B126 and B136, suggesting that they played a minor role, at best, in the functions of that group. A majority of the infrequent synapse classes are due to the presynaptic involvements of only 4 cell types, PHA, PHC, PVN and DVB, which contact many less active cell types. PHA and PHC each form several frequent synapse classes, while DVB forms 1 and PVN forms no frequent synapse class. DVB and PVN also make synapses to hypodermal cells (see Table 3). Thus, the choice of postsynaptic partners by some neurons may be relatively non-specific and promiscuous, in contrast to the rules governing the formation of the frequent synapse classes. The PHA data in Table 3 demonstrate this tendency rather clearly, as their many postsynaptic targets do not fall into any highly repeated groups.
The behavioral role of the infrequent synapse classes is unclear. Walthall and Chalfie (1988) have suggested that certain classes of chemical synapse may have no behavioral function in C. elegans but are formed as a byproduct of an axonal guidance mechanism and, thereafter, retained to preserve the relative position of the presynaptic processes within a fiber bundle. However, in their example, the synapses from AVM-> BDU are actually rather frequent in the rostral portion of the ventral cord. Another possibility is that some neurons function in a way that requires only that they have a rather diffuse output, not a specific one. As an example, they might function in a relatively non-specific modulatory way, providing general neuronal activation or deactivation. Such neuromodulation might be effective at a distance from the presynaptic release sites, influencing many neurons which are not in direct contact (Barchas et al., 1978). This would allow infrequent synapse classes to be somewhat variable while still maintaining a rationale for their existence and for their concentration within a few presynaptic cell types.
Developmental noise The infrequent synapse classes formed by cells other than PVN, PHA, PHC, or DVB are quite widely distributed among a number of pre- and postsynaptic participants. Almost all of them are nonreproducible, and they usually contain 1, sometimes 2, contacts per class. It seems unlikely that they are of behavioral importance; instead, they seem to us to represent developmental "noise" of some sort. One possibility is that they represent the residue of synapse classes that were more frequent at an earlier developmental stage. One instance of developmental rewiring, involving the loss of some synapses and the gain of others, has been reported for C. elegans (White et al., 1978). Another possibility is that infrequent synapse classes represent a secondary consequence of whatever mechanisms operate to establish the frequent synapse classes. This would be an example of developmental noise as described by Waddington (1957). Macagno et al. (1973) noted a similar background scatter of infrequent connections in the Daphnia optic lamina, where each receptor cell forms frequent synapses (20-80 contacts) onto a particular lamina cell and a lesser or equal number of contacts onto unidentified processes. Besides these frequent synapses, much smaller numbers of synapses go to other lamina cells or even to other receptor cell processes. Some of these infrequent synapses also appear to be obeying patterns, but some others, perhaps a few percent of the total, are sufficiently unusual to indicate that they represent developmental noise. The well-studied development of the optic lamina makes it unlikely that this noise represents the residue of previously frequent synapse classes.
The existence of developmental noise of the Waddington type seems reasonable in evolutionary terms. If selection during evolution is simply for appropriate function, and if this function is carried out principally by the frequent synapse classes, then there seems to be little reason why programs evolved to establish the frequent classes should undergo further refinement, probably at additional informational cost, simply to eliminate a minority of behaviorally unimportant synapses.
Combinatorial specification of synapse development. A reproducible pattern among the frequent synapse classes has been noted in the preanal ganglion in 3 animals (Tables 4, 5). It is intriguing to note that (1) these synapses are almost all dyadic, (2) bilateral homologues are rarely contacted simultaneously, (3) several different dyadic combinations occur at different frequencies, and (4) many possible combinations of synaptic partners never occur. The combinatorial aspects of this pattern are particularly striking, suggestive of the requirement for simultaneous recognition of different postsynaptic recognition factors from 2 separate partners before a synapse can be formed. Mutant alleles that fail to form many chemical synapses are already known to be viable in C. elegans (Hall et al., 1989). Mutations of synaptic recognition factors are likely to produce viable alleles as well, probably having an uncoordinated phenotype. Currently, more than 100 unc genes have been isolated as viable mutant alleles and mapped on the genome (see Wood, 1988). Thus, the preanal ganglion presents a model system in which to explore the genetics of synapse specification
|