This muscle is sexually dimorphic between hermaphrodite and male. In males, the contractile apparatus of the anal depressor detaches from the dorsal hypodermis and attaches to the dorsal spicule protractor during the L4 molt, completely reorienting the myofilaments to run anteroposteriorly instead of dorsoventrally (Male-specific Muscles) (Sulston and Horvitz, 1977; Sulston et al., 1980; White, 1988).
5 Anal Sphincter Muscle
This is a single cell with a toroidal region encircling the proximal part of the rectum and about eight thin processes that radiate medially and laterally from this toroid. These include four long, filament-filled processes that extend laterally to anchor to the body wall on the dorsal and ventral sides (MusFIG 18 and MusFIG 20). The anal sphincter muscle circles the intestine at its junction with the rectum. During defecation, it is dilated before enteric muscle contractions occur to allow waste material to pass into the rectum. It then contracts nearly simultaneously with the other enteric muscles, possibly helping to squeeze the posterior intestine for expulsion of the waste (Reiner and Thomas, 1995; Avery and Thomas, 1997). The toroidal part of the muscle contains a continuous ring of contractile filaments, many of which do not seem to connect to any significant end-point attachment structures. In this aspect, it is thought to be similar to vertebrate smooth muscle (White, 1988). Filaments within the medially projecting, short processes occasionally show electron-dense attachments to the gland cells at the roof and floor of the rectal passageway (MusFIG 20) (see also Alimentary System - Intestine and Rectum and Anus).

This muscle is sexually dimorphic between hermaphrodite and male. In males, near the end of the L4 stage the muscle goes under a dramatic hypertrophy during the opening of a cloacal canal. In contrast to hermaphrodites and larval males, this modified sphincter must relax in males to permit defecation (see Male-specific Muscles) (Reiner and Thomas, 1995; Emmons and Sternberg, 1997).
6 Vulval Muscles
The two sets of vulval muscles are vm1 and vm2, and each set contains four muscle cells with single sarcomeres (MusFIG 21). Both vulval and uterine muscles in the hermaphrodite are born at the late-L3 stage from two sex myoblasts, M.vlpaa and M.vrpaa. Through three consecutive divisions, each sex myoblast generates two sets (vm and um) of four daughters, with a total of 16 cells located around the developing vulva by the L3 molt. Eight of these comprise the vulval muscles. The four vm1 muscles insinuate between rows of ventral body wall muscles and run between the dorsal edge of the ventral body wall muscle quadrant and the vulC and vulD toroids of vulva (White et al., 1986). The four vm2 muscles run between the ventral margin of the body wall muscle quadrants and the uterus–vulva junction (between the uterus and vulF toroid). Hence, vm1 cells attach to the vulva more ventrally than do vm2 and are more superficial (Sulston and Horvitz, 1977). The single sarcomeres of each vulval muscle stretch along the entire muscle length and attach through hemiadherens junctions to discrete zones in the body wall on one end and to the vulval epithelium and vulval cuticle on the opposite end around the late-L4 stage (White, 1988). The nuclei of the sex muscles also settle into their final positions around the developing gonad at the L4 stage. Among vulval muscles, the vm2s are the only ones that are directly innervated by the VC and HSN neurons of the egg-laying circuitry. The other muscles are either directly or indirectly connected to vm2 by gap junctions (see also Gap Junctions). vm2s send muscle arms to a local neuropil on either side of the hypodermal ridge to receive synaptic input (White, 1988). Coordinated contraction of the vulval muscles expands the uterus and pulls the vulval lips apart, opening the passage for eggs to be expelled (Hodgkin, 1988) (see also Reproductive System - Egg-laying apparatus). Nematodes missing all eight vulval muscles are unable to lay eggs (Bird and Bird, 1991).
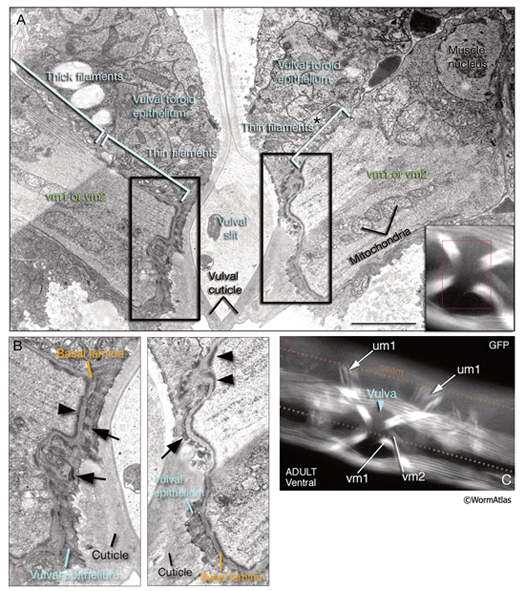
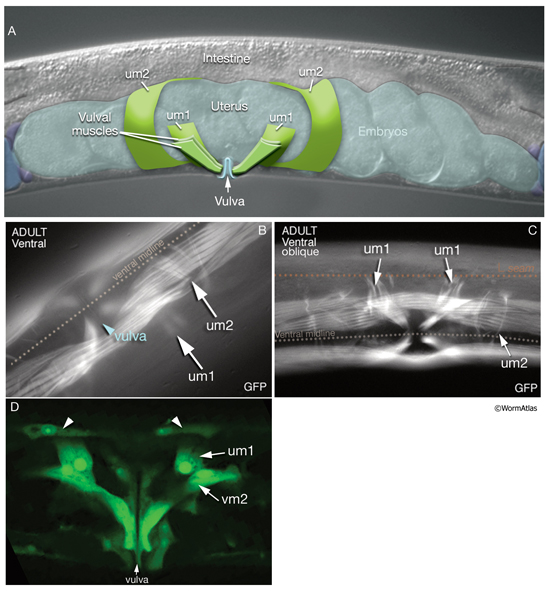
7 Uterine Muscles
There are two sets of uterine muscles, um1 and um2, with four post-embryonically born muscle cells in each set. Each um1 cell makes a quarter of a circle that cups the proximal uterus on the ventral side and attaches to it close to the vulva (MusFIG 22). Dorsally, these cells attach to the lateral seam cells close to the utse-seam attachment sites (Vogel and Hedgecock, 2001; Woo et al., 2004). In each half (left and right sides) uterus, the distal sets of uterine muscle cells (um2) make half circles that wrap around the uterus and attach to it in a region further from the vulva (Sulston and Horvitz, 1977). The filaments of the uterine muscles are circumferentially oriented, which, when the muscle contracts, may move eggs through the uterus by a squeezing action (Sulston and Horvitz, 1977). This myofilament network seems to be anchored to the thin basal lamina on the surface facing the uterus by randomly placed attachment points, similar to the distribution of the dense bodies in vertebrate smooth muscles (MusFIG 1D). There is no direct innervation of the uterine muscles. Instead, they are coupled via gap junctions to vulval muscles (White et al., 1986) (see also Reproductive System - Egg-laying apparatus and Gap Junctions). Nematodes missing all eight uterine muscles are still able to lay eggs, as has been shown by ablation studies (Bird and Bird, 1991).
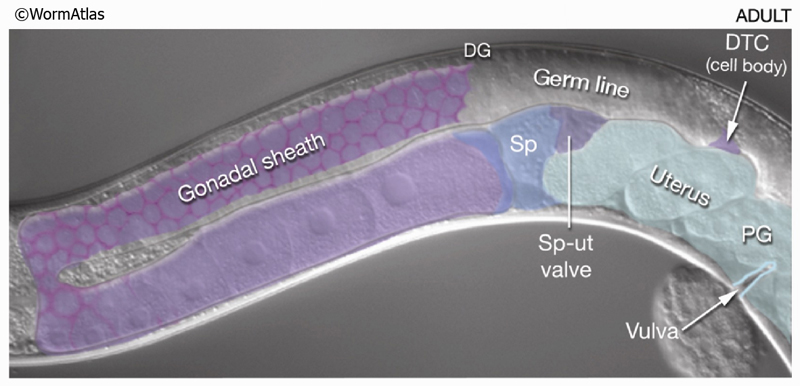
8 Contractile Gonad Sheath of the Hermaphrodite
Five pairs of gonadal sheath cells have stereotyped positions along the proximal–distal axis of the gonad and cover the germ-line tissue of each gonadal arm (see MusFIG 17 and Reproductive System - Somatic gonad). Sheath cell pairs 3, 4, and 5 abundantly express muscle filament components such as actin and myosin that are organized into dense networks (Hirsh et al., 1976; Strome, 1986; Goetinck and Waterston, 1994; McCarter et al., 1997; Hall et al., 1999). Filaments are predominantly longitudinally oriented in pairs 3 and 4 and both longitudinally and circumferentially oriented in pair 5 (MusFIG 18). Filaments are also present in sheath cells 1 and 2, but are much less abundant. The myofilament network of the contractile gonadal sheath is possibly similar in organization to that of vertebrate smooth muscle. Anchorage points for the myofilament lattice are distributed diffusely over the outward-facing cell surface. Each connects to the nearby basal lamina by an electron-dense attachment on the plasma membrane. During ovulation, contraction of the proximal sheath pulls the dilated spermatheca over the most proximal oocyte and hence transfers this oocyte into the spermatheca for fertilization. The sheath is not innervated. Instead, it contracts periodically, possibly in response to recurrent intracellular Ca++ transient currents (Bui and Sternberg, 2002, and references therein) (see Reproductive System - Somatic gonad). Gap junctions connect the sheath cells to one another, and transitory gap junctions connect the sheath to the primary oocyte (Hall et al., 1999) (see also Gap Junctions). Thus, signals between the germ line and sheath may coordinate sheath contractions to events in the oocyte and to the presence of sperm (McCarter et al., 1999; Miller et al., 2001; 2003).
MusFIG 24: Distal and proximal gonadal sheath cells have different characteristics.A. Epifluorescent image showing the anterior gonad arm of a lim-7::GFP transgenic adult hermaphrodite, lateral view, left side. Five pairs of sheath cells (numbered 1-5) cover the arm. The LIM-7::GFP signal is strong in sheath-cell pairs 1-3, weak in 4, and absent from 5. The approximate position of sheath-cell borders is indicated by the dotted line. (N) Nucleus; (PG) proximal gonad; (DG) distal gonad. Magnification, 400x. (Strain source: O. Hobert.) B. Epifluorescent image of an adult (dissected) gonad arm costained with DAPI (blue, cell nuclei) and rhodamine phalloidin (red, actin). Magnification, 400x. (Image source: D. Killian and E.J. Hubbard.)
9 List of Nonstriated Muscle Cells
i. Pharyngeal muscles (pm); Note that in earlier publications these cells are labeled as "m"; m1, m2, m3 etc., here they are labeled "pm" for "pharyngeal muscle" (Avery L. and Thomas J. H., 1997)
1. First pharyngeal muscle ring; all fuse into one syncytium around hatching
pm1DL
pm1DR
pm1L
pm1R
pm1VL
pm1VR
2. Second pharyngeal muscle ring
pm2DL; fuses with DR around hatching
pm2DR; fuses with DL around hatching
pm2L; fuses with VL around hatching
pm2R; fuses with VR around hatching
pm2VL; fuses with L around hatching
pm2VR; fuses with R around hatching
3. Third pharyngeal muscle ring
pm3DL; fuses with DR around hatching
pm3DR; fuses with DL around hatching
pm3L; fuses with VL around hatching
pm3R; fuses with VR around hatching
pm3VL; fuses with L around hatching
pm3VR; fuses with R around hatching
4. Fourth pharyngeal muscle ring
pm4DL; fuses with DR around hatching
pm4DR; fuses with DL around hatching
pm4L; fuses with VL around hatching
pm4R; fuses with VR around hatching
pm4VL; fuses with L around hatching
pm4VR; fuses with R around hatching
5.Fifth pharyngeal muscle ring
pm5DL; fuses with DR around hatching
pm5DR; fuses with DL around hatching
pm5L; fuses with VL around hatching
pm5R; fuses with VR around hatching
pm5VL; fuses with L around hatching
pm5VR; fuses with R around hatching
6. Sixth pharyngeal muscle ring
pm6D
pm6VL
pm6VR
7. Seventh pharyngeal muscle ring
pm7D
pm7VL
pm7VR
8. Eighth pharyngeal muscle ring
pm8
ii. Enteric muscles
1. Stomatointestinal muscle
mu intL; ABplpppppaa
mu intR; MSppaapp
2. Anal sphincter muscle
mu sph; ABprpppppap
3. Anal depressor muscle
mu anal; ABplpppppap
iii. Hermaphrodite sex muscles
1. vm1
M.vrpaapaa
M.vrpaaapp
M.vlpaapaa
M.vlpaaapp
2. vm2
M.vrpaapap
M.vrpaaapa
M.vlpaapap
M.vlpaaapa
3. um1
M.vrpaappa
M.vrpaaaap
M.vlpaappa
M.vlpaaaap
3. um2
M.vrpaappp
M.vrpaaaaa
M.vlpaappp
M.vlpaaaaa
iv. Hermaphrodite contractile gonadal sheath
1. Somatic Sheath (10 cells/5 pairs) of anterior gonad arm:
Z1.apa (sheath cell 1)
Z1.appaaa (sheath cell 2)
Z1.appaap (sheath cell 3)
Z1.appapa (sheath cell 4)
Z1.appapp (sheath cell 5)
Z1.paaa (sheath cell 1)
Z1.paapaaa (sheath cell 2)
Z1.paapaap (sheath cell 3)
Z1.paapapa (sheath cell 4)
Z1.paapapp (sheath cell 5)
2. Somatic Sheath (10 cells/5 pairs) of posterior gonad arm:
Z4.pap (sheath cell 1)
Z4.paappp (sheath cell 2)
Z4.paappa (sheath cell 3)
Z4.paapap (sheath cell 4)
Z4.paapaa (sheath cell 5)
Z4.appp (sheath cell 1)
Z4.appappp (sheath cell 2)
Z4.appappa (sheath cell 3)
Z4.appapap (sheath cell 4)
Z4.appapaa (sheath cell 5)
10 References
Albertson, D.G. and Thomson, J.N. 1976. The pharynx of Caenorhabditis elegans. Phil. Trans. Royal Soc. London 275B: 299-325. Article
Avery D.G. and Thomas, J.H. 1997. Feeding and defecation. In C. elegans Volume II. Ed.s Riddle D.L., Blumenthal, T., Meyer B.J. and Priess J.R . Pp 679-716. Cold Spring Harbor Laboratory Press. Article
Bird, A.F. and Bird, J. 1991. The structure of nematodes. Academic Press, San Diego, CA.
Bui, Y.K. and Sternberg, P.W. 2002. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol. Biol. Cell 13: 1641-1651. Article
Emmons, S.W. and Sternberg, P.W. 1997. Male Development and Mating Behavior. In C. elegans II (ed. D. L. Riddle et al.). Chapter 12. pp.295-334. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Article
Goetinck, S. and Waterston, R.H. 1994. The Caenorhabditis elegans muscle-affecting gene unc-87 encodes a novel thin filament-associated protein. J .Cell Biol. 127: 79-93. Article
Hall, D. H., Winfrey, V. P., Blaeuer, G., Hoffman, L. H., Furuta, T., Rose, K. L., Hobert, O., Greenstein, D. 1999. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 212: 101-123. Article
Hirsh, D., Oppenheim, D. and Klass, M. 1976. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49: 200-219. Abstract
Hodgkin, J. 1988. Sexual dimorphism and sex determination. In "The nematode C. elegans" (W. B. Wood ed.) pp243-280. Cold Spring Harbor Laboratory Press, New York. Abstract
Kimble, J. and Hirsh, D. 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396-417. Article
McCarter, J., Bartlett, B., Dang, T., and Schedl, T. 1997. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181: 121-143. Article
McCarter, J., Bartlett, B., Dang, T., and Schedl, T. 1999. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205: 111-128. Article
McKay, S.J., Johnsen, R., Khattra, J., Asano, J., Baillie, D.L., Chan, S., Dube, N., Fang, L., Goszczynski, B., Ha, E., Halfnight, E., Hollebakken, R., Huang, P., Hung, K., Jensen, V., Jones, S.J.M., Kai, H., Li, D., Mah, A., Marra, M., McGhee, J., Newbury, R., Pouzyrev, R., Riddle, D.L., Sonnhammer, E., Tian, H., Tu, D., Tyson, J.R., Vatcher, G., Warner, A., Wong, K., Zhao, Z. and Moerman, D.G. 2004. Gene expression profiling of cells, tissues and developmental stages of the nematode C. elegans. Cold Spring Harbor Symp. Quantit. Biol. 68: 159-69. Abstract
Miller, M.A., Nguyen, V.Q., Lee, M.H., Kosinski, M., Schedl, T., Caprioli, R.M. and Greenstein, D. 2001. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291: 2144-2147. Abstract
Miller, M.A., Ruest, P.J., Kosinski, M., Hanks, S.K. and Greenstein, D. 2003. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 17: 187-200. Article
Moerman, D.G. and Fire, A. 1997. Muscle: Structure, Function, and Development. In C. elegans Volume II. Ed.s Riddle D.L., T Blumenthal, BJ Meyer and JR Priess. Pp 417-470. Cold Spring Harbor Laboratory Press. Article
Reiner, D.J. and Thomas, J.H. 1995. Reversal of a muscle response to GABA during C. elegans male development. J. Neurosci. 15: 6094-6102. Article
Strome, S. 1986. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J. Cell Biol. 103: 2241-2152. Article
Sulston, J.E. and Horvitz, H.R. 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. Article
Sulston, J.E., Albertson, D.G. and Thomson, J.N. 1980. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev Biol. 78: 542-576. Article
Thomas, J.H. 1990. Genetic analysis of defecation in Caenorhabditis elegans. Genetics 124: 855-872. Article
Vogel, B.E. and Hedgecock, E.M. 2001. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128: 883–894. Article
White, J.G., Southgate, E., Thomson, J.N. and Brenner, S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. Roy. Soc. Lond. 314B: 1-340. Article
White, J. 1988. The Anatomy. In The nematode C. elegans (ed. W.B. Wood). Chapter 4. pp 81-122. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Abstract
Woo, W.M., Goncharov, A., Jin, Y. and Chisholm, A.D. 2004. Intermediate filaments are required for C. elegans epidermal elongation. Dev. Biol. 267: 216-229. Article
|