|
Methods to Post Stain Thin Sections
by Hall, Hartwieg and Nguyen
doi:10.3908/wormatlas.9.11
Overview
We have previously described methods for staining grids in bulk using a dental wax device to hold 50 grids at once (Hall, 1995). Here, we describe methods for staining single grids by hand and an automated machine to stain many grids at a time.
For most EM work, grids containing thin sections need to be counterstained with heavy metals, most often with uranyl acetate and/or lead citrate. The time, concentration and temperature of these staining steps depend on the embedding resin, tissue density, and section thickness. Even for worms that have been prepared in a similar fashion, certain individual specimens will stain faster or slower than others. For this reason, we usually begin by staining just a few grids, and then examine them under the TEM to decide upon a best protocol for the rest of the grids from that experiment. In working with immunoEM protocols, one should avoid using lead citrate as a counter stain, since artifactual lead stain has a similar appearance to immunogold particles.
Single Grid Staining
While this method is slower and more labor intensive than staining grids in bulk, it allows for the greatest freedom in tailoring the final contrast for a particular specimen. In fact, since a given animal may stain rather differently from one portion of the thin section series to another, we often change the stain protocol for specific regions of the anatomy.
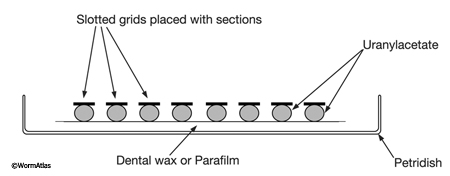
Place a piece of Parafilm (or dental wax) in a covered Petri dish. Then place several drops of uranyl acetate (one drop for each grid) on the substrate and put grids upside down onto each drop, so that the tissue contacts the staining fluid (EMPoststainFIG 1). Cover the dish during staining and place a piece of foil over the cover to keep the samples dark. After the staining step is complete, remove the grid with a forceps and wash it for about 3 min. by moving the grid up and down in a beaker filled with distilled water. Then place the grids into another Petri dish with drops of lead citrate if desired. It is important that several pellets of NaOH are positioned within the Petri dish near the staining drops to prevent excessive precipitation of lead citrate. Again, keep dish covered during staining.
 EMPoststainFIG 1 EMPoststainFIG 1
Helpful Hints
Staining is done at room temperature on the benchtop. Since stains may change subtly as they age, they should generally be kept refrigerated and protected from light when not in use. We always filter each stain immediately before use. It is often convenient to store a few milliliters of stain in a plastic syringe attached to 0.22 mm filter, wrapped in foil, so that a fresh filtered drop can be delivered when needed.
Digital EM cameras allow one to achieve high contrast while using less staining, since the camera itself provides more contrast (gain) than one could easily achieve in the dark room. In fact, the digital image contains many more gray levels than a silver halide image on film, so subtle differences in contrast can sometimes be accentuated (see below). The poststaining protocol used for a study using EM film may require longer stain times and/or more concentrated stain than when shooting digital images.
Stain Recipes for Uranyl Acetate
Under various circumstances, we have tried many different stains and achieved good results. For instance, we have used UAc as an aqueous stain, or as an alcoholic stain in 100% ethanol or 70% methanol. Alcoholic stains work much faster, but can
be difficult to control in a reproducible fashion. Stock solutions of UAc can be prepared in a mildly acidic buffer (sodium acetate or veronal acetate) at pH 5.2, or in distilled water. Aqueous stains can be controlled very well, but can be quite slow to act. Here are some typical combinations:
| Aqueous stains: |
1% UAc in dH2O
2% UAc in dH2O
4% UAc in dH2O |
10 to 45 min
5 to 20 min
5 to 15 min |
| Alcoholic stains: |
2% UAc in 100% ethanol
2% UAc in 70% methanol
4% UAc in 70% methanol
|
1 to 2 min
5 to 15 min
2 to 10 min |
| Mixtures: |
one drop 2% UAc in 100% ethanol + one drop dH2O |
5 min |
Helpful Hints
Staining thicker sections for electron tomography requires longer staining times. For 250 nm sections, we have been using 4% UAc in dH2O for 80100 min. Others have tried warming this stain to make it work faster.
Troubleshooting
Artifacts caused by excess UAc stain are uncommon. They typically take the appearance of thin needle-like crystals on top of the tissue, or as a ring of these crystals encircling the tissue, just below the cuticle layer. Such artifacts can be caused by failing to filter the stain just before use, or by failing to rinse the tissue both before and after staining with the correct pH buffer. Switching from a UAc stain directly back into a pH 7 buffer can cause massive precipitation of the stain.
Within the past decade, there has also been a change in the formulation of the uranium source for UAc crystals that seems to have altered the chemistry of UAc stains. The aim of the chemical suppliers was to change the potential radionuclide hazard in the uranium salt, but the result seems to have included a change in the chemical activity. Thus, many UAc protocols have been adjusted to longer stain times or more concentrated solutions since 2000.
Stain Recipes for Lead Citrate
Our experience with lead citrate staining shows that one can often utilize more dilute stain for C. elegans compared to mammalian tissues (Hall, 1995). Instead of using Reynolds lead stain according to the usual recipe (Reynolds, 1963), we recommend diluting the stain in water by 1/10, 1/100, or even 1/1000 to control its rate of staining. One can store the diluted stain at 4°C for a month in the dark (or in a brown bottle), and one can continue to make new dilutions from the stock bottle of Reynolds lead stain for many months. However, lead citrate should always be filtered immediately before use to remove any precipitates.
Troubleshooting
This stain can be difficult to control, which is one principal reason for diluting it before use. Overstaining with lead citrate is a common problem, which results in dense black speckling over the tissue. One should not use lead citrate in combination with immunoEM methods, since lead artifact can be confused with small gold particles.
Machine Staining of Grids
Use of a staining machine can produce highly reproducible results. Since the stain solutions are stored air-tight and light-tight inside metallic pouches they are less likely to degrade or precipitate while in storage. Some staining machines can be programmed for a range of times and temperature; thus, nematode sections may use a different protocol than mammalian cells. For C. elegans embedded in a medium hard Epon-Araldite mixture, good results have been achieved on the Leica Ultrastainer with 4560 min at 4°C for the UAc stain, followed by a 10 min wash, and then 20 min stain at room temperature for lead citrate (using Leica-prepared solutions in each case), followed by another 15 min wash. Washing time should be longer after the lead citrate than for UAc. The special flexible device that comes with the autostainer holds about 50 grids.
Helpful Hints
Besides Leica, another vendor for such equipment is Electron Microscopy Sciences (EMS; Hatfield, PA).
Figures
 Click pictures for new window with figure and legend, click again for high resolution image Click pictures for new window with figure and legend, click again for high resolution image
EMPoststainFIG 1: Staining grids on Parafilm. Each grid floats on a separate droplet of freshly filtered stain. The staining dish is kept closed by a cover (not shown) to prevent drying. NaOH pellets (not shown) are placed inside the closed dish near the staining drops when using lead citrate to prevent excess lead precipitate.
References
Hall, D.H. 1995. Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol. 48: 395-436. Abstract
Hall, D.H., Hartweig, E. and Nguyen, K.C.Q. 2012. Modern electron microscopy methods for C. elegans. Methods Cell Biol. 107: 93-149. Abstract
Reynolds, E.S. 1963. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. J. Cell Biol. 17: 208. Article
| 